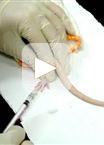
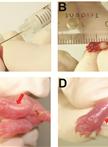
An Experimental Model of Neonatal Nociceptive Stimulation in Rats
In order to survive, preterm and/or sick neonates need diagnostic and therapeutic measures that may cause discomfort, stress and pain during a critical period of intense growing and modeling of the central nervous system (Anand et al., 2013). Scientific interest in the long lasting effects of the Neonatal Intensive Care (NIC) experience, which provides a sensory experience completely different from the natural uterine environment, is growing (Jobe, 2014). The follow-up of critically ill newborn infants until adulthood indicated an association of early noxious stimuli with long lasting alterations in somatosensory and cognitive processing (Doesburg, 2013; Vinall et al., 2014; Vinall et al., 2013). However, one major limitation of the clinical studies is the difficulty to distinguish between long-term effects of pain suffered during neonatal intensive care and other confounding factors such as the presence of non-painful stress during hospital stay, the occurrence of acute and chronic morbidities, the post-natal environmental influences and family care. In this context, the understanding of the roles played by each factor and the interplay between these diverse variables require the use of animal models. The protocol described here is used to model the noxious stimulation in which premature newborns are subjected during treatment in the NIC. The current protocol models inflammatory nociceptive stimulation in neonatal rats, as previously demonstrated (Leslie et al., 2011; Lima et al., 2014; Malheiros et al., 2014). Complete Freund's adjuvant (CFA) is a solution of antigen emulsified in mineral oil and used as an immunopotentiator, causing a painful reaction that lasts 7-8 days after subcutaneous injection. It is effective in stimulating cell-mediated immunity. The rodent model of neonatal inflammatory stimulation with CFA is advantageous because at birth the formation of the central nervous system is incomplete in rat pups and corresponds to that of 24 week intra-uterine human preterm neonates (Anand et al., 1999), following similar patterns in the development of the pain system (Fitzgerald and Anand,1993). The first postnatal week in newborn rat pups corresponds to human premature infants from 24-36 weeks of gestation (Kim et al., 1996; Wilson, 1995), offering a suitable condition to model and compare preterm (rat pups on P1) to full term (rat pups on P8) infants subjected to noxious stimulation. In this paper, we present our methods to induce nociceptive inflammatory stimulation in neonatal rat pups as an attractive approach to study short- or long-term effects and the mechanisms underlying the behavioral repertoire of ex-premature infants or adolescents.