- Submit a Protocol
- Receive Our Alerts
- EN
- Protocols
- Articles and Issues
- About
- Become a Reviewer
Past Issue in 2017
Volume: 7, Issue: 16
Cancer Biology
Mouse Model of Dextran Sodium Sulfate (DSS)-induced Colitis
Teratoma Formation Assay for Assessing Pluripotency and Tumorigenicity of Pluripotent Stem Cells
Rapid Profiling Cell Cycle by Flow Cytometry Using Concurrent Staining of DNA and Mitotic Markers
Antisense Oligonucleotide-mediated Knockdown in Mammary Tumor Organoids
Cell Biology
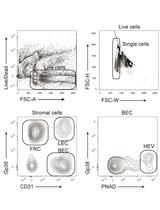
Isolation and Analysis of Stromal Cell Populations from Mouse Lymph Nodes
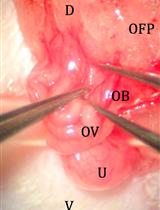
Improved Oviduct Transfer Surgery for Genetically Modified Rat Production
Immunology
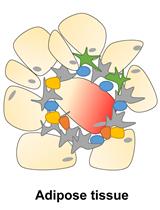
Isolation and Analysis of Stromal Vascular Cells from Visceral Adipose Tissue
Establishment of a Human Cell Line Persistently Infected with Sendai Virus
Macrophage Survival Assay Using High Content Microscopy
Microbiology
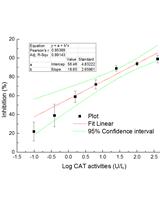
Superoxide Dismutase (SOD) and Catalase (CAT) Activity Assay Protocols for Caenorhabditis elegans
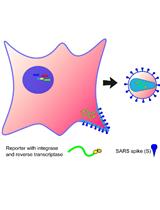
An Optimized Method for the Production Using PEI, Titration and Neutralization of SARS-CoV Spike Luciferase Pseudotypes
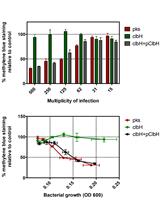
Protocol for HeLa Cells Infection with Escherichia coli Strains Producing Colibactin and Quantification of the Induced DNA-damage
Detection of Pathogens and Ampicillin-resistance Genes Using Multiplex Padlock Probes
Snapshots of the Signaling Complex DesK:DesR in Different Functional States Using Rational Mutagenesis and X-ray Crystallography
Molecular Biology
Assessment of Modulation of Protein Stability Using Pulse-chase Method
Neuroscience
Assessment of Thermal Pain Sensation in Rats and Mice Using the Hargreaves Test
A High-throughput Assay for mRNA Silencing in Primary Cortical Neurons in vitro with Oligonucleotide Therapeutics
An ex vivo Perifusion Method for Quantitative Determination of Neuropeptide Release from Mouse Hypothalamic Explants
Plant Science
Quantification of Membrane Damage/Cell Death Using Evan’s Blue Staining Technique
TUNEL Assay to Assess Extent of DNA Fragmentation and Programmed Cell Death in Root Cells under Various Stress Conditions
Using Silicon Polymer Impression Technique and Scanning Electron Microscopy to Measure Stomatal Aperture, Morphology, and Density
ROS Detection in Botryococcus braunii Colonies with CellROX Green Reagent
Stem Cell
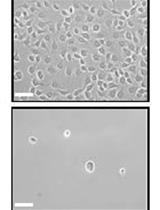
Isolation and Expansion of Mesenchymal Stem Cells from Murine Adipose Tissue
Exit from Pluripotency Assay of Mouse Embryonic Stem Cells
A Co-culture Assay to Determine Efficacy of TNF-α Suppression by Biomechanically Induced Human Bone Marrow Mesenchymal Stem Cells