- Submit a Protocol
- Receive Our Alerts
- EN
- Protocols
- Articles and Issues
- About
- Become a Reviewer
Past Issue in 2018
Volume: 8, Issue: 22
Biochemistry
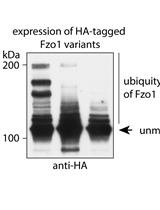
Separation and Visualization of Low Abundant Ubiquitylated Forms
Cancer Biology
A Functionally Robust Phenotypic Screen that Identifies Drug Resistance-associated Genes Using 3D Cell Culture
Cell Biology
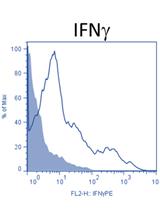
In-vivo gp100-specific Cytotoxic CD8+ T Cell Killing Assay
Relative Quantitation of Polymerized Actin in Suspension Cells by Flow Cytometry
Developmental Biology
Electron Microscopic Detection of Proteins and Protein Complexes in Mammalian Cells Using APEX-tagged, Conditionally Stable Nanobodies
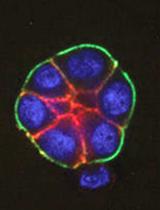
Mosaic Labeling and 3-Dimensional Morphological Analysis of Single Cells in the Zebrafish Left-right Organizer
Environmental science
Microplastic Extraction from Marine Vertebrate Digestive Tracts, Regurgitates and Scats: A Protocol for Researchers from All Experience Levels
Microbiology
Use of Gas Chromatography to Quantify Short Chain Fatty Acids in the Serum, Colonic Luminal Content and Feces of Mice
Neuroscience
Collagenase-based Single Cell Isolation of Primary Murine Brain Endothelial Cells Using Flow Cytometry
Papain-based Single Cell Isolation of Primary Murine Brain Endothelial Cells Using Flow Cytometry
Purification of Soluble Recombinant Human Tau Protein from Bacteria Using Double-tag Affinity Purification
A Modified Barnes Maze for Juvenile Rats
Stem Cell
One-step Derivation of Functional Mesenchymal Stem Cells from Human Pluripotent Stem Cells