- Submit a Protocol
- Receive Our Alerts
- EN
- Protocols
- Articles and Issues
- About
- Become a Reviewer
Past Issue in 2020
Volume: 10, Issue: 3
Biophysics
Evaluating Whole Blood Clotting in vitro on Biomaterial Surfaces
Cancer Biology
Identification of Target Protein for Bio-active Small Molecule Using Photo-cross Linked Beads and MALDI-TOF Mass Spectrometry
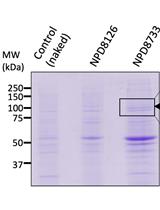
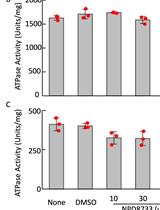
Measurement of ATPase Activity of Valosin-containing Protein/p97
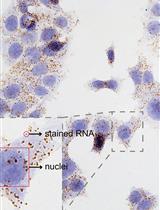
Detection of Individual RNA in Fixed Cells and Tissues by Chromogenic ISH
Approaching RNA-seq for Cell Line Identification
Cell Biology
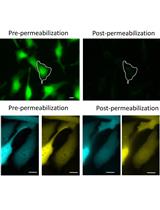
Live Mitochondrial or Cytosolic Calcium Imaging Using Genetically-encoded Cameleon Indicator in Mammalian Cells
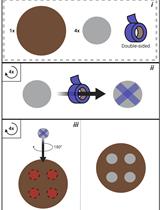
Rapid Nickel-based Isolation of Extracellular Vesicles from Different Biological Fluids
Developmental Biology
Mouse Embryonic Tooth Germ Dissection and Ex vivo Culture Protocol
Microbiology
Expression and Purification of Adeno-associated Virus Virus-like Particles in a Baculovirus System and AAVR Ectodomain Constructs in E. coli
Rapid Detection of Proliferative Bacteria by Electrical Stimulation
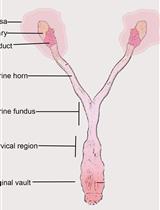
Transcervical Mouse Infections with Chlamydia trachomatis and Determination of Bacterial Burden
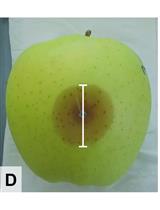
Competition Assays to Quantify the Effect of Biocontrol Yeasts against Plant Pathogenic Fungi on Fruits
Molecular Biology
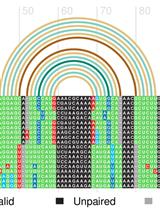
Structural Alignment and Covariation Analysis of RNA Sequences
Neuroscience
Implanting and Recycling Neuropixels Probes for Recordings in Freely Moving Mice
Stem Cell
In vitro Self-organized Mouse Small Intestinal Epithelial Monolayer Protocol