- Submit a Protocol
- Receive Our Alerts
- EN
- Protocols
- Articles and Issues
- About
- Become a Reviewer
Past Issue in 2020
Volume: 10, Issue: 7
Biochemistry
Measurement of Protein-Protein Interactions through Microscale Thermophoresis (MST)
Quantitative Determination of Ca2+-binding to Ca2+-sensor Proteins by Isothermal Titration Calorimetry
Preparation, FPLC Purification and LC-FT-ICR-MS of Proteins
Spectrophotometric Assessment of Heme Oxygenase-1 Activity in Leishmania-infected Macrophages
Determination of Flavin Potential in Proteins by Xanthine/Xanthine Oxidase Method
Cancer Biology
In vivo Tumor Growth and Spontaneous Metastasis Assays Using A549 Lung Cancer Cells
Cell Biology
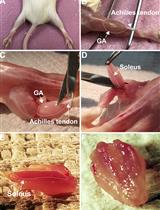
Isolation, Purification and Characterization of Exosomes from Fibroblast Cultures of Skeletal Muscle
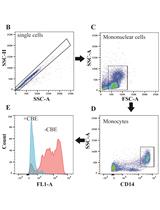
Flow Cytometry Measurement of Glucocerebrosidase Activity in Human Monocytes
Fractionation of Ovarian Follicles and in vitro Oocyte Maturation and Ovulation Assay of Ciona intestinalis Type A
Developmental Biology
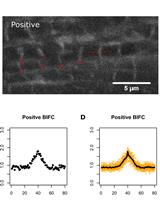
Bimolecular Fluorescence Complementation (BiFC) for Studying Sarcomeric Protein Interactions in Drosophila
Microbiology
Conjugation Protocol Optimised for Roseburia inulinivorans and Eubacterium rectale
Molecular Biology
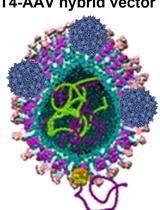
Preparation of a Bacteriophage T4-based Prokaryotic-eukaryotic Hybrid Viral Vector for Delivery of Large Cargos of Genes and Proteins into Human Cells
Real-time Fluorescence Measurement of Enterovirus Uncoating
Measuring cAMP Specific Phosphodiesterase Activity: A Two-step Radioassay
Neuroscience
A Simple and Fast Battery Test for Phenotypic Characterization of Mice











.jpg)






.jpg)