- Submit a Protocol
- Receive Our Alerts
- EN
- Protocols
- Articles and Issues
- About
- Become a Reviewer
Past Issue in 2020
Volume: 10, Issue: 11
Biochemistry
Biochemical Pulldown of mRNAs and Long Noncoding RNAs from Cellular Lysates Coupled with Mass Spectrometry to Identify Protein Binding Partners
Isolating Multiple Extracellular Vesicles Subsets, Including Exosomes and Membrane Vesicles, from Bovine Milk Using Sodium Citrate and Differential Ultracentrifugation
Cell Biology
Extracellular Vesicles Tracking and Quantification Using CT and Optical Imaging in Rats
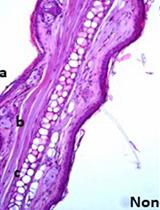
Ex vivo Culture Assay Using Human Hair Follicles to Study Circadian Characteristics
Developmental Biology
FRET Reporter Assays for cAMP and Calcium in a 96-well Format Using Genetically Encoded Biosensors Expressed in Living Cells
Primed Track: Reliable Volumetric Single-cell Tracking and Lineage Tracing of Living Specimen with Dual-labeling Approaches
Immunology
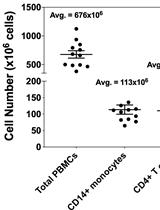
Methodology for in vitro Assessment of Human T Cell Activation and Blockade
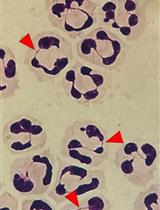
Isolation and High Throughput Flow Cytometric Apoptosis Assay of Human Neutrophils to Enable Compound Library Screening
RNA Extraction from Ears and Draining Lymph Nodes of Mice Infected with Leishmania amazonensis
Microbiology
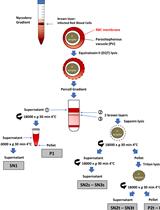
Differential Fractionation of Erythrocytes Infected by Plasmodium berghei
Molecular Biology
Mouse Adipose Tissue Protein Extraction
Neuroscience
Enriched Environment Procedures for Rodents: Creating a Standardized Protocol for Diverse Enrichment to Improve Consistency across Research Studies
Precise Targeting of Single Microelectrodes to Orientation Pinwheel Centers
Plant Science
Safe DNA-extraction Protocol Suitable for Studying Tree-fungus Interactions
Determination of Ureides Content in Plant Tissues