- Submit a Protocol
- Receive Our Alerts
- EN
- Protocols
- Articles and Issues
- About
- Become a Reviewer
Past Issue in 2021
Volume: 11, Issue: 20
Biochemistry
Co-immunofluorescence of MRPL12 and Nrf2 in HK2 Cells
Biophysics
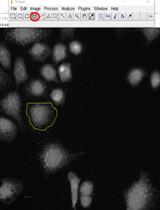
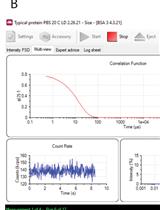
Measurement of the Translational Diffusion Coefficient and Hydrodynamic Radius of Proteins by Dynamic Light Scattering
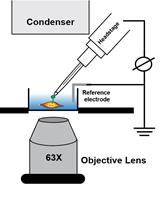
Electrophysiological Recordings of the Polycystin Complex in the Primary Cilium of Cultured Mouse IMCD-3 Cell Line
Biophysical Characterization of Iron-Sulfur Proteins
Cancer Biology
Urea Denaturation, Zinc Binding, and DNA Binding Assays of Mutant p53 DNA-binding Domains and Full-length Proteins
Cell Biology
Collection of in vivo Capacitated Sperm from Different Locations Along the Reproductive Tract of Time-Mated Female Mice by Microdissection
Developmental Biology
Isolation and Culturing Primary Cholangiocytes from Mouse Liver
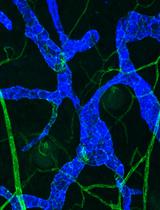
Whole-mount Immunohistochemistry to Visualize Mouse Embryonic Dermal Lymphatic Vasculature
Microbiology
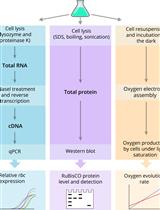
Quantification of RuBisCO Expression and Photosynthetic Oxygen Evolution in Cyanobacteria
A Rapid Induction Overexpression System for the Fission Yeast Schizosaccharomyces pombe
Neuroscience
Isolation and Electrophysiology of Murine Sympathetic Postganglionic Neurons in the Thoracic Paravertebral Ganglia
Isolation and Phospholipid Enrichment of Muscle Mitochondria and Mitoplasts
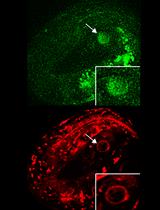
Simultaneous Monitoring Cytoplasmic Calcium Ion and Cell Surface Phosphatidylserine in the Necrotic Touch Neurons of Caenorhabditis elegans
Plant Science
High Throughput Analyses of Ascorbate-turnover Enzyme Activities in Rice (Oryza sativa L.) Seedlings
High-throughput Screening for Defense Priming-inducing Compounds in Parsley Cell Cultures
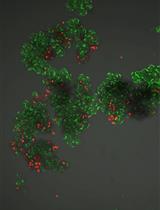
Proteoliposomes for Studying Lipid-protein Interactions in Membranes in vitro