- Submit a Protocol
- Receive Our Alerts
- EN
- Protocols
- Articles and Issues
- About
- Become a Reviewer
Past Issue in 2022
Volume: 12, Issue: 0
Biochemistry
Assay to Study the Phase-transition Behavior of Edc3, a Conserved Processing Body (P-body) Marker Protein
Biological Engineering
Co-differentiation and Co-maturation of Human Cardio-pulmonary Progenitors and Micro-Tissues from Human Induced Pluripotent Stem Cells
Cancer Biology
A Fast and Reliable Method to Generate Pure, Single Cell-derived Clones of Mammalian Cells
In-Cell Western Protocol for Semi-High-Throughput Screening of Single Clones
Cell Biology
A Semi-quantitative Scoring System for Green Histopathological Evaluation of Large Animal Models of Acute Lung Injury
Preparation of a Single-cell Suspension from Drosophila Wing Imaginal Discs
Immunology
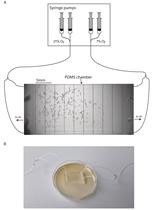
Combination of Sterile Injury and Microbial Contamination to Model Post-surgical Peritoneal Adhesions in Mice
Medicine
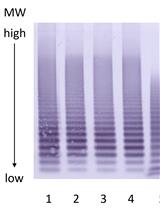
Von Willebrand Factor Multimer Analysis by Low Resolution SDS-Agarose Gel Electrophoresis
Neuroscience
Aerotaxis Assay in Caenorhabditis elegans to Study Behavioral Plasticity
Plant Science
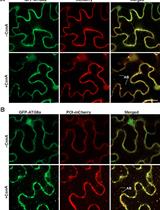
Colocalization Assay with Fluorescent-tagged ATG8 Using a Nicotiana benthamiana-based Transient System