- Submit a Protocol
- Receive Our Alerts
- EN
- Protocols
- Articles and Issues
- About
- Become a Reviewer
Past Issue in 2015
Volume: 5, Issue: 19
Cell Biology
Sample Preparation for Correlative Light and Electron Microscopy (CLEM) Analyses in Cellular Microbiology
Immunology
TCRβ Clonotype Analysis of EBV and CMV-specific Human CD8+ T Cells
Microbiology
Density Gradient Centrifugation for Enrichment and Identification of GFP-tagged Chitosomal Microvesicles of Filamentous Fungi
Bacterial Porphyrin Extraction and Quantification by LC/MS/MS Analysis
In vitro Studies: Inhibition of Nevirapine Metabolism by Nortriptyline in Hepatic Microsomes
Neuroscience
Chick Neural Tube Explant Culture
Plant Science
3’ Rapid Amplification of cDNA Ends (3’ RACE) Using Arabidopsis Samples
Quantification of Callose Deposition in Plant Leaves
Hydroponic Culture of ‘Micro-Tom’ Tomato
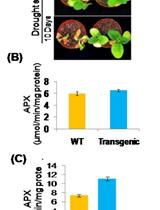
Pot Level Drought Stress Tolerance Assay in Tobacco through Plant Phenotyping and Antioxidant Assay
Histochemical Staining of Silica Body in Rice Leaf Blades
Pectin Nanostructure Visualization by Atomic Force Microscopy
Analysis of in vivo Cellulose Biosynthesis in Arabidopsis Cells by Spinning Disk Confocal Microscopy