- Submit a Protocol
- Receive Our Alerts
- EN
- Protocols
- Articles and Issues
- About
- Become a Reviewer
Past Issue in 2023
Volume: 13, Issue: 23
Biochemistry
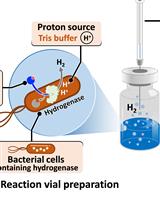
H2 Production from Methyl Viologen–Dependent Hydrogenase Activity Monitored by Gas Chromatography
Cell Biology
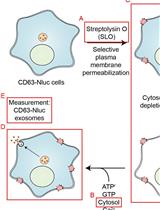
Biochemical Reconstitution of Ca2+-Dependent Exosome Secretion in Permeabilized Mammalian Cells
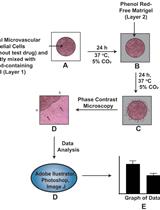
An Improved Protocol for the Matrigel Duplex Assay: A Method to Measure Retinal Angiogenesis
Developmental Biology
A Methodology for the Enzymatic Isolation of Embryonic Hypothalamus Tissue and Its Acute or Post-Culture Analysis by Multiplex Hybridisation Chain Reaction
Immunology
Microinjection of β-glucan Into Larval Zebrafish (Danio rerio) for the Assessment of a Trained-Like Immunity Phenotype
Microbiology
Purification of Human Cytoplasmic Actins From Saccharomyces cerevisiae
Molecular Biology
Expression and Purification of Recombinant Human Mitochondrial RNA Polymerase (POLRMT) and the Initiation Factors TFAM and TFB2M
Neuroscience
Habituation of Sugar-Induced Proboscis Extension Reflex and Yeast-Induced Habituation Override in Drosophila melanogaster
Targeted Delivery of Chemogenetic Adeno-Associated Viral Vectors to Cortical Sulcus Regions in Macaque Monkeys by Handheld Injections
Systems Biology
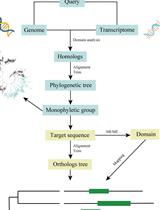
Phylogenetic Inference of Homologous/Orthologous Genes among Distantly Related Plants