- Submit a Protocol
- Receive Our Alerts
- EN
- Protocols
- Articles and Issues
- About
- Become a Reviewer
Past Issue in 2015
Volume: 5, Issue: 23
Cancer Biology
Human Blood Component Vaccinia Virus Neutralization Assay
Cell Biology
Single Molecule RNA FISH in the Mammalian Oocyte
Immunology
Isolation of Lymphocytes from Murine Visceral Adipose Tissue
Excision of Visceral Adipose Tissue from Live Mice (VATectomy)
Microbiology
Establishing a Biofilm Co-culture of Pseudomonas and Aspergillus for Metabolite Extraction
The Application of Quercetin to Study the Effect of Hsp70 Silencing on Plant Virus Infection in Nicotiana benthamiana Plants
Plant Science
Terminal Restriction Fragments (TRF) Method to Analyze Telomere Lengths
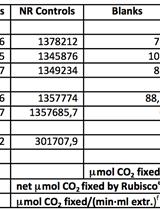
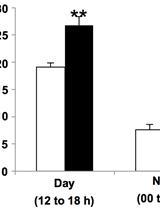
Purification of Rubisco from Chlamydomonas reinhardtii
Quantification of the Volume and Surface Area of Symbiosomes and Vacuoles of Infected Cells in Root Nodules of Medicago truncatula
Analysis of Developing Pollen Grains within Intact Arabidopsis thaliana Anthers by Olympus Two-Photon Laser Scanning Microscopy
Assay of the Carboxylase Activity of Rubisco from Chlamydomonas reinhardtii
Chlorophyll Content Assay to Quantify the Level of Necrosis Induced by Different R Gene/Elicitor Combinations after Transient Expression
Measurement of H+ Flux in Rice by Non-invasive Micro-test Technology
Extraction of Intracellular and Cell Wall Proteins from Leaves and Roots of Harsh Hakea
Rhizosphere Acidification Assay