- Submit a Protocol
- Receive Our Alerts
- EN
- Protocols
- Articles and Issues
- About
- Become a Reviewer
Past Issue in 2016
Volume: 6, Issue: 3
Cancer Biology
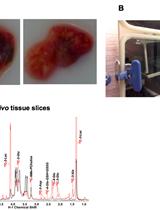
Stable Isotope Resolved Metabolomics Studies in ex vivo TIssue Slices
Cell Biology
Mouse Oocyte Isolation, Cultivation and RNA Microinjection
Immunology
Mouse BMDC-dependent T Cell Polarization Assays
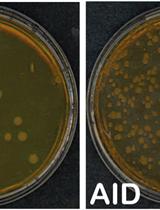
Cell-based Assays to Monitor AID Activity
Microbiology
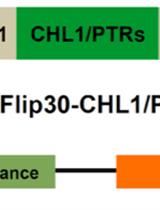
Design and Functional Analysis of Fluorescent Nitrate and Peptide Transporter Activity Sensors in Yeast Cultures
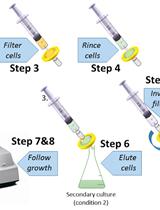
Calculation of Microorganism Lag Times as a Measure of Adaptative Capability between Different Growth Conditions
Neuroscience
Craniotomy for Cortical Voltage-sensitive Dye Imaging in Mice
Plant Science
Structured Illumination Microscopy (SIM) and Photoactivated Localization Microscopy (PALM) to Analyze the Abundance and Distribution of RNA Polymerase II Molecules on Flow-sorted Arabidopsis Nuclei
Quantification of Ethylene Production in Tomato Leaves Infected by Xanthomonas euvesicatoria
Preparation of Chloroplast Lipid Membrane and Lipid-protein Interaction Assay
Measurement of PI4P Levels in Intact Chloroplasts Isolated from Arabidopsis thaliana
Stem Cell
Transfection of Embryoid Bodies with miRNA Precursors to Induce Cardiac Differentiation