Improve Research Reproducibility A Bio-protocol resource
- Submit a Protocol
- Receive Our Alerts
- EN
- Protocols
- Articles and Issues
- About
- Become a Reviewer
Past Issue in 2016
Volume: 6, Issue: 4
Biochemistry
RAB21 Activity Assay Using GST-fused APPL1
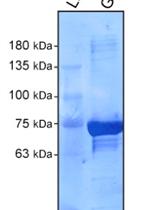
The Rab family of small GTPases are essential regulators of membrane trafficking events. As with other small GTPase families, Rab GTPases cycle between an inactive GDP- bound state and an active GTP-bound state. Guanine nucleotide exchange factors (GEFs) promote Rab activation with the exchange of bound GDP for GTP, while GTPase-activating proteins (GAPs) regulate Rab inactivation with GTP hydrolysis. Numerous methods have been established to monitor the activation status of Rab GTPases. Of those, FRET-based methods are used to identify when and where a Rab GTPase is activated in cells. Unfortunately, the generation of such probes is complex, and only a limited number of Rabs have been probed this way. Biochemical purification of activated Rabs from cell or tissue extracts is easily achievable through the use of a known Rab effector domain to pull down a specific GTP-bound Rab form. Although this method is not ideal for detailed subcellular localization, it can offer temporal resolution of Rab activity. The identification of a growing number of specific effectors now allows tests for activation levels of many Rab GTPases in specific conditions. Here, we described an affinity purification approach using GST fused APPL1 (a known RAB21 effector) to test RAB21 activation in mammalian cells. This method was successfully used to assay changes in RAB21 activation status under nutrient rich versus starved conditions and to test the requirement of the MTMR13 RAB21 GEF in this process.
Cancer Biology
In vitro Flow Adhesion Assay for Analyzing Shear-resistant Adhesion of Metastatic Cancer Cells to Endothelial Cells
Hematogenous metastasis is a primary cause of mortality from metastatic cancer. The shear-resistant adhesion of circulating tumor cells to the vascular endothelial cell surface under blood flow is an essential step in cell extravasation and further tissue invasion. This is similar to a process exploited by leukocytes for adhesion to inflamed blood vessels (leukocyte mimicry). The shear resistant adhesion is mediated by high affinity interactions between endothelial adhesion molecules and their counter receptor ligand expressed on circulating cells. Thus, weak interaction results in a rapid detachment of circulating cells from endothelium. Despite the critical role of vascular adhesion of cancer cells in hematogenous metastasis, our knowledge regarding this process has been limited due to the difficulty of mimicking dynamic flow conditions in vitro. In order to gain better insight into the shear-resistant adhesion of cancer cells to the endothelium, we developed a protocol for measuring the shear resistant adhesion of circulating tumor cells to endothelial cells under physiologic flow conditions by adapting a well established flow adhesion assay for inflammatory cells. This technique is useful to evaluate 1) the shear resistant adhesion competency of cancer cells and 2) the endothelial adhesion molecules necessary to support cancer cell adhesion (Kang et al., 2015).
Cell Biology
VAMP8-3xHA Uptake Assay in HeLa Cells
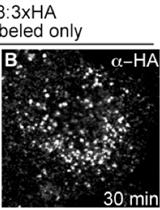
Transmembrane proteins are rarely exclusively localized to a specific vesicle or an organelle. Most transmembrane proteins undergo complicated trafficking routes. Thus, transmembrane proteins are under constant flux, and at steady state, found on a variety of vesicles or organelles. This characteristic makes the study of their trafficking routes complex, since at any given moment, different molecules are often being trafficked in opposing directions. Pulse-chase experiments can temporally track a specific pool of a transmembrane protein of interest, allowing for the kinetic description of its trafficking route. This type of technique has been used extensively to follow a large array of plasma membrane localized proteins (Diril et al., 2006; Jean et al., 2010). Here, we describe a method that allows the study of VAMP8 trafficking from the plasma membrane to endolysosomal compartments. This method was used to describe a role for MTMR13 and RAB21 in the regulation of VAMP8 trafficking to endolysosomes (Jean et al., 2015).
Immunology
Isolation of Nippostrongylus brasiliensis Larvae from Mouse Lungs
The rodent parasite Nippostrongylus brasiliensis (N. brasiliensis) models the salient features of helminth infection including skin penetration, migration from tissues to lung, maturation and egg production in the gut. As a potent activator of systemic and mucosal Th2 immune responses, Nippostrongylus brasiliensis has been extensively used to study host protective immunity and in vivo regulation of Th2 immune response. Six to eight week old C57Bl/6J, Balb/c mice or any other strains are suitable, as all are susceptible to infection. Inocula of 150-650 L3 larvae can be administered by subcutaneous injection, but for greatest consistency a dose of 550 L3 larvae is routinely used for experimental purposes. We have optimized three different protocols for the isolation of larvae from the lungs of mice infected with the L3 stage of Nippostrongylus brasiliensis. Larvae can migrate to the lung between 18-60 h post inoculation from any site in the body. The numbers of larvae appearing in the lung peaks at 48 h after inoculation and it is recommended that isolation/harvesting be performed at 48 h for greatest consistency of each harvest method:
Dye Labeling of Live Nippostrongylus brasiliensis Larvae for Visualization in Host Tissue
Visualization of the interaction between parasitic nematodes and their host enables a better understanding of the development of the nematode during the infectious stages of its life cycle and of the effects of host response on nematode integrity in tissues. Appropriate live imaging of these nematode/host interactions, to date has been hindered by the lack of appropriate molecular tools or efficient labeling agents. Here, we present techniques for the live labeling of the nematode parasite Nippostrongylus brasiliensis (N. brasiliensis) that allows visualization of the parasite in the mouse host for up to 24 h. The external sheath can be labeled with CFSE allowing infective larvae to be identified and followed until the stage of exsheathment. The internal labeling of infective parasites can be performed by ingestion of NY microspheres. The worms can continue to be identified for up to 24 h following exsheathment. This should be applicable to other parasitic nematodes.
Neuroscience
Synaptoneurosome Preparation from C57BL/6 Striata
Activity-dependent local mRNA translation endows synapses to remodel their structure and function (Bramham and Wells, 2007). This process is tightly controlled by the state of phosphorylation of several components of the translational machinery including initiation factors and ribosomal proteins (Buffington et al., 2014). The present protocol describes a method to prepare striatal synaptoneurosomes, from adult mice, containing both pre- and postsynaptic elements in which the level of synaptic phospho-proteins can be quantified (Biever et al., 2015).
Repeated Cross-fostering Protocol as a Mouse Model of Early Environmental Instability
Early life events have a crucial role in programming the individual phenotype indeed the exposure to traumatic experiences during infancy can increase later risks for a variety of neuropsychiatric conditions, including mood and anxiety disorders. Several studies in rodents demonstrated the impact of short and long sessions of separation/isolation from caregivers in developing pups, on the behavioral and hormonal response to stress during infancy and adulthood (D’Amato et al., 1998; Meaney et al., 2000; Luchetti et al., 2015). The repeated cross-fostering (RCF) is an early manipulation carried out in mouse pups during the first four postnatal days life. Differently from other early manipulations, hypotalamic-pituitary-adrenal (HPA) axis functioning is not altered in RCF treated subjects. This manipulation is used to model human early environmental instability, a risk factor for internalizing disorders including separation anxiety disorder, panic disorder and CO2 hypersensitivity (Kendler et al., 1992; Forman and Davies, 2003; Battaglia et al., 2009).
Stem Cell
Immunofluorescent Staining of Mouse Intestinal Stem Cells
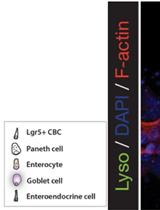
Immunofluorescent staining of organoids can be performed to visualize molecular markers of cell behavior. For example, cell proliferation marked by incorporation of nucleotide (EdU), or to observe markers of intestinal differentiation including paneth cells, goblet cells, or enterocytes (see Figure 1). In this protocol we detail a method to fix, permeabilize, stain and mount intestinal organoids for analysis by immunofluorescent confocal microscopy.Figure 1. A schematic depicting a crypt-villus forming organoid, and visualization of Paneth cells by immunofluorescence staining. Left: Small intestinal organoids grow as crypt-villus structures that contain all of the multiple differentiated lineages of the intestine. Right: Immunofluorescent staining can be used to visualize individual cell types in the organoid. Here paneth cells are visualized by staining for lysozyme (“Lyso,” Green), which reveals Paneth cells located at crypt bases. F-Actin (Red) reveals crypt structure at the apical surface of the epithelium, and DAPI (Blue) reveals cell nuclei. Scale bar is 25 μm.
Isolation, Culture, and Maintenance of Mouse Intestinal Stem Cells
In this protocol we describe our modifications to a method to isolate, culture and maintain mouse intestinal stem cells as crypt-villus forming organoids. These cells, isolated either from the small or large intestine, maintain self-renewal and multilineage differentiation potential over time. This provides investigators a tool to culture wild type or transformed intestinal epithelium, and a robust assay for stem cell tissue homeostasis in vitro.