- Submit a Protocol
- Receive Our Alerts
- EN
- Protocols
- Articles and Issues
- About
- Become a Reviewer
Past Issue in 2016
Volume: 6, Issue: 22
Biochemistry
Ionization Properties of Phospholipids Determined by Zeta Potential Measurements
Determining Efficiency and Selectivity of Lipid Extraction by Perturbing Agents from Model Membranes
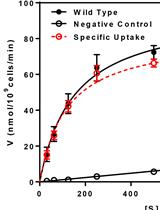
Determination of Rate of [3H-methyl]-choline Incorporation into Cellular Lipids and Non-lipid Metabolites
Cancer Biology
Establishment of Patient-Derived Xenografts in Mice
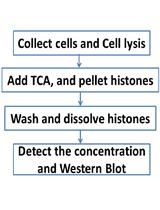
Acid Extraction of Total Histone from Colon Cancer HCT116 Cells
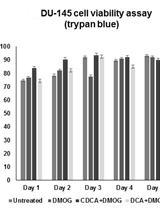
Determining the Influence of Small Molecules on Hypoxic Prostate Cancer Cell (DU-145) Viability Using Automated Cell Counting and a Cell Harvesting Protocol
Cell Biology
Quantitative Measurements of HIV-1 and Dextran Capture by Human Monocyte-derived Dendritic Cells (MDDCs)
Immunology
Isolation of Highly Pure Primary Mouse Alveolar Epithelial Type II Cells by Flow Cytometric Cell Sorting
Evaluation of Cross-presentation in Bone Marrow-derived Dendritic Cells in vitro and Splenic Dendritic Cells ex vivo Using Antigen-coated Beads
Determination of H2O2 Generation by pHPA Assay
Assay to Evaluate BAL Fluid Regulation of Fibroblast α-SMA Expression
Analysis of Phagosomal Antigen Degradation by Flow Organellocytometry
Microbiology
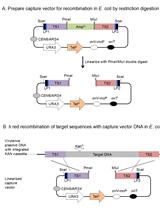
Transfer of Large Contiguous DNA Fragments onto a Low Copy Plasmid or into the Bacterial Chromosome
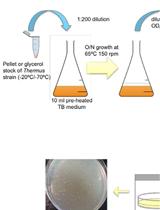
Transformation of Thermus Species by Natural Competence
Heterologous Expression and Purification of the Magnesium Transporter A (MgtA) in Escherichia coli
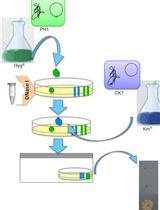
Cell-to-cell DNA Transfer among Thermus Species
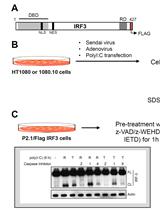
Biochemical Analysis of Caspase-8-dependent Proteolysis of IRF3 in Virus-infected Cells
Halo Assay for Toxic Peptides and Other Compounds in Microorganisms
Uptake Assay for Radiolabeled Peptides in Yeast
Neuroscience
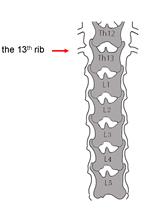
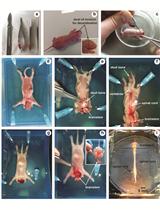
Microinjection of Virus into Lumbar Enlargement of Spinal Dorsal Horn in Mice
In vitro Brainstem-spinal Cord Preparation from Newborn Rat
An in vitro Model of Neuron-macrophage Interaction to Generate Macrophages with Neurite Outgrowth Properties
Plant Science
Arabidopsis Seed Germination Assay with Gibberellic Acid
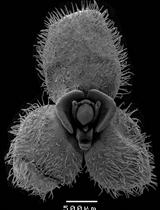
Documentation of Floral Secretory Glands in Pleurothallidinae (Orchidaceae) Using Scanning Electron Microscopy (SEM)
Cryopreservation Protocol for Chlamydomonas reinhardtii
Visualising Differential Growth of Arabidopsis Epidermal Pavement Cells Using Thin Plate Spline Analysis
Stem Cell
Isolation and Primary Culture of Various Cell Types from Whole Human Endometrial Biopsies
Isolation and Culture of Human Adipose-derived Stem Cells from Subcutaneous and Visceral White Adipose Tissue Compartments




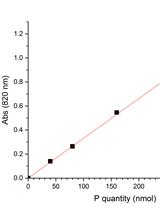


![Determination of Rate of [3H-methyl]-choline Incorporation into Cellular Lipids and Non-lipid Metabolites](https://en-cdn.bio-protocol.org/imageup/arcimg/20161108094711249.jpg?t=1758534059)