- Submit a Protocol
- Receive Our Alerts
- EN
- Protocols
- Articles and Issues
- About
- Become a Reviewer
Past Issue in 2013
Volume: 3, Issue: 22
Cell Biology
Harvest and Culture of Mouse Peritoneal Macrophages
Immunology
Gastric Aspiration Models
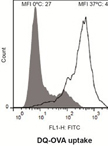
Assessment of Human Dendritic Cell Antigen Uptake by Flow Cytometry
Immunocytochemical Detection of Recombinant Biomphalysin on Schistosoma mansoni Sporocysts
Microbiology
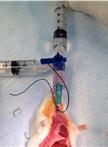
Assay to Evaluate Vascular Permeability Induction in Mice
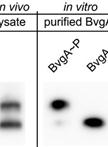
Separation and Detection of Phosphorylated and Nonphosphorylated BvgA, a Bordetella pertussis Response Regulator, in vivo and in vitro
Shigella IpaD and IpaB Surface Localizations
Transport Assays in Aspergillus nidulans
Neuroscience
Cell Cycle Analysis in the Vertebrate Brain Using Immunolabeled Fresh Cell Nuclei
Plant Science
Analysis of RNA-protein Interactions Using Electrophoretic Mobility Shift Assay (Gel Shift Assay)
Bimolecular Fluorescence Complementation (BIFC) Protocol for Rice Protoplast Transformation
Shikimate Hydroxycinnamoyl Transferase (HCT) Activity Assays in Populus nigra
Binding Assay of Cytosolic Proteins to the Cytoskeleton
Mapping and Analysis of Illumina Reads for Transcriptome of Medicago Truncatula During the Early Organogenesis of the Nodule
Cotton Ovules Culture and Analysis