- Submit a Protocol
- Receive Our Alerts
- EN
- Protocols
- Articles and Issues
- About
- Become a Reviewer
Past Issue in 2013
Volume: 3, Issue: 24
Cancer Biology
Invadopodia Detection and Gelatin Degradation Assay
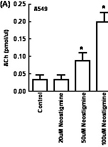
Measurement of Acetylcholine from Cell Lines
Cell Biology
Autophagy Assays (LC3B immunofluorescence, LC3B western blot, acridine orange assay)
ImmunoFISH for Adherent Cultured Mammalian Cells
ImmunoFISH for Mice and Baboons Frozen Sections
Immunology
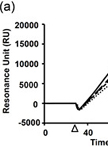
Surface Plasmon Resonance Analysis of Antigen-Antibody Interaction
Influenza Virus-cell Fusion Inhibition Assay
Isolation of Neutralizing Antibody
Microbiology
EMS Mutagenesis of Clostridium difficile to Identify Strains with Germination-null Phenotypes
Colony Forming Assay for HCV-Replicon Cell Line
Virulence Studies of Clostridium difficile
Neuroscience
Ex utero Electroporation into Mouse Embryonic Neocortex
Plant Science
Membrane Preparation, Sucrose Density Gradients and Two-phase Separation Fractionation from Five-day-old Arabidopsis seedlings
Library Construction for Genome-wide Bisulfite Sequencing in Plants
Observation of Chloroplast-actin Filaments in Leaves of Arabidopsis
Stem Cell
Preparation of Adult Mouse Muscle Stem Cells
Intestinal Differentiation of Mouse ESCs
Intestinal Differentiation of Human ESCs