- Submit a Protocol
- Receive Our Alerts
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Thin Sections of Technovit 7100 Resin of Rice Endosperm and Staining
Published: Vol 4, Iss 18, Sep 20, 2014 DOI: 10.21769/BioProtoc.1239 Views: 13769
Reviewed by: Tie LiuAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

A Protocol for Mitotic Metaphase Chromosome Count Using Shoot Meristematic Tissues of Mulberry Tree Species
Raju Mondal [...] K. Vijayan
Sep 5, 2023 387 Views

Synthetic Promoter Screening Using Poplar Mesophyll Protoplast Transformation
Yongil Yang [...] C. Neal Stewart Jr.
Apr 20, 2023 573 Views
An In-depth Guide to the Ultrastructural Expansion Microscopy (U-ExM) of Chlamydomonas reinhardtii
Nikolai Klena [...] Virginie Hamel
Sep 5, 2023 1042 Views
Abstract
Starch is a biologically and commercially important carbohydrate that is accumulated in plant storage organs, such as seed endosperm. Starch is water-insoluble and forms transparent grains, referred to as starch grains (SGs). SGs are easily stained by iodine solution and can be observed under a normal microscope. Technovit 7100 resin is suitable for preparation of thin sections from endosperm. The thin sections and iodine staining can visualize SGs clearly inside the endosperm cell. This protocol provides the procedures to prepare thin sections of Technovit 7100 resin from rice endosperm. It can also be applicable to the seeds of other plant species.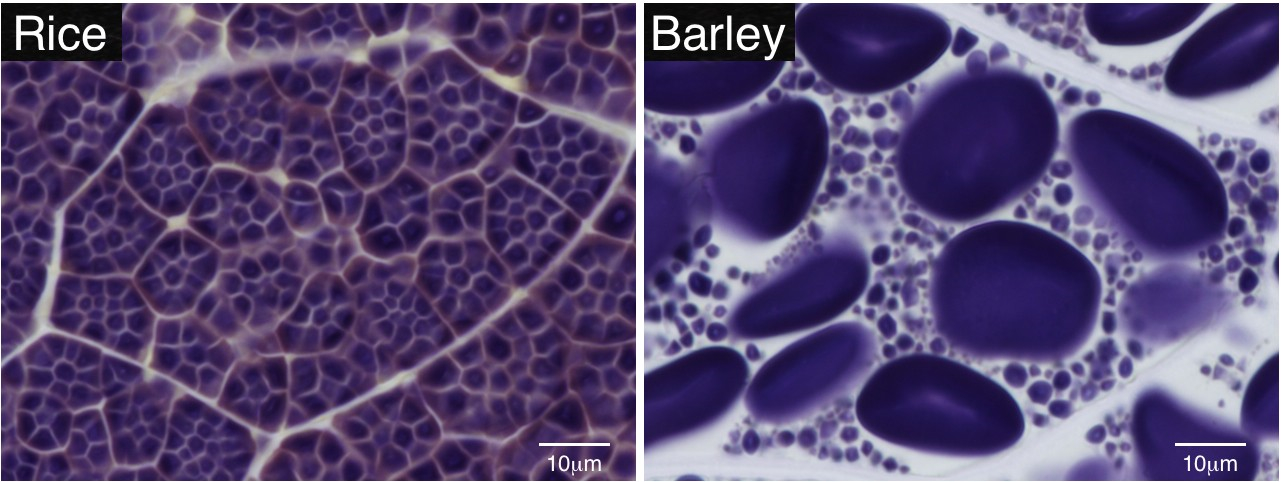
Figure 1. Iodine-stained Technovit thin sections of matured endosperm cells in rice and barley. Starch grains (SGs) can be visualized as violet particles.
Materials and Reagents
- Rice seeds
- Technovit 7100 resin (Heraeus Kulzer)
- Lugol solution (MP Biomedicals, catalog number: 155269 )
- Formalin
- Acetic acid
- 99.5% ethanol (commercially available one)
- Instant adhesives (Aron alpha, Toagosei Co.)
- FAA solution (see Recipes)
- 100% ethanol (see Recipes)
- 100% Technovit 7100 (I) (see Recipes)
- 25% Technovit 7100 resin (I) (see Recipes)
- 50% Technovit 7100 resin (I) (see Recipes)
- 75% Technovit 7100 resin (I) (see Recipes)
- 100% Technovit 7100 (II) (see Recipes)
Equipment
- Silanized slides (Dako, catalog number: S3003 )
- Wooden dowels (6 x 25 mm)
- Razor blade (Feather, catalog number: FH-10 )
- 1.5 ml plastic tube
- 0.2 ml PCR tube
- Molecular sieves 4A 1/16 (Nacalai tesque, catalog number: 04172-95 )
- Ultramicrotome (Leica Microsystems, model: EM UC7 )
- Diamond knife (Diatome AG, Ultra trim, 3.0 mm)
- Rotator (TAITEC, model: RT-30mini )
- Forceps (Vigor, stainless steel #5)
- Aspirator (Ulvac, model: MDA-015 )
- Microscope (OLYMPUS, model: AX70 )

Figure 2. A wooden dowel
Procedure
- Resin embedding of endosperm
- Cut out approximately 1 mm cubic blocks from the center region of the endosperm in brown rice seeds using razor blades.
- Put the blocks into an empty 1.5-ml plastic tube.
- Add 500 µl of FAA solution into the plastic tube. The blocks should be sunken at the bottom of tube.
- Make the tube open and place it in the aspirator and vacuum for 5 min.
- Release the negative pressure gently.
- Repeat steps 4 and 5 two more time.
- Take the tube from the aspirator and remove the FAA by micropipette.
- Add 1 ml of fresh FAA solution into the tube.
- Rotate at 30 r/min overnight at room temperature.
- Remove FAA and add 1 ml of 30% ethanol.
- Rotate at 30 r/min for 1 h at room temperature.
- Remove 30% ethanol and add 1 ml of 50% ethanol.
- Rotate at 30 r/min for 1 h at room temperature.
- Remove 50% ethanol and add 1 ml of 70% ethanol.
- 15. Rotate at 30 r/min for 1 h at room temperature.
- Remove 70% ethanol and add 1 ml of 90% ethanol.
- Rotate at 30 r/min for 1 h at room temperature.
- Remove 90% ethanol and add 1 ml of 95% ethanol.
- Rotate at 30 r/min for 1 h at room temperature.
- Remove 95% ethanol and add 1 ml of 100% ethanol.
- Rotate at 30 r/min for 1 h at room temperature.
- Exchange 100% ethanol and rotate at 30 r/min for 1 h.
- Exchange 100% ethanol and rotate at 30 r/min overnight at room temperature.
- Exchange 100% ethanol and rotate at 30 r/min for 1 h at room temperature.
- Remove 100% ethanol and add 1 ml of 25% Technovit 7100 resin (I) Rotate at 30 r/min for 1 h at room temperature.
- Remove 25% Technovit 7100 resin (I) and add 1 mL of 50% Technovit 7100 resin (I).
- Rotate at 30 r/min for 1 h at room temperature.
- Remove 50% Technovit 7100 resin (I) and add 1 ml of 75% Technovit 7100 resin (I).
- Rotate at 30 r/min for 1 h at room temperature.
- Remove 75% Technovit 7100 resin (I) and add 1 ml of 100% Technovit 7100 resin (I).
- Rotate at 30 r/min for 1 h at room temperature.
- Exchange 100% Technovit 7100 resin (I) and rotate at 30 r/min for 1 h at room temperature.
- Exchange 100% Technovit 7100 resin (I) and rotate at 30 r/min overnight at room temperature.
- Exchange 100% Technovit 7100 resin (I) and rotate at 30 r/min for 1 h at room temperature.
- Remove 100% Technovit 7100 resin (I) from the tube at room temperature.
- Transfer the endosperm block into the 0.2-ml PCR tube. One PCR tubes should contain one endosperm block.
- Add 150-200 µl of 100% Technovit 710 (II) in the PCR tube.
- Confirm that the endosperm block precipitate on the bottom of the PCR tube. If floated, try to send to the bottom by pipetting.
- Incubate the PCR-tube at 37 °C for more than 2 day to solidify the resin.
- Cut out approximately 1 mm cubic blocks from the center region of the endosperm in brown rice seeds using razor blades.
- Trimming the solidified resin
- Remove the PCR tube from the solidified resin by razor blades.
- Trim the solidified resin by razor blades to remove the extra resin. Leave intact the part where the endosperm block is embedded.
- Adhere the trimmed resin on the wooden dowel by instant super adhesives.
- Remove the PCR tube from the solidified resin by razor blades.
- Thin sectioning and staining
- Fix the wooden dowel on the sample holder of ultramicrotome to make the resin facing outward.
- Set the diamond knife on the ultramicrotome.
- Adjust the angles, directions and positions of the dowel and diamond knife to make the knife-edge can approach at appropriate directions to the endosperm blocks in the resin.
- Start sectioning of resin by preceding the position of diamond knife by 1 µm each.
- Continue the sectioning of resin to obtain the thin sections containing endosperm blocks.
- Prepare a drop of water on a silanized slide.
- Pick up the thin section containing endosperm blocks by forceps.
- Put the thin section onto the surface of water drop on the slide. The thin section will expand on the surface of water.
- Leave the thin section overnight at room temperature to dry out the water.
- Add a drop of 40-times diluted Lugol solution on the dried thin section stuck on the slide.
Note: It takes 1-5 sec to stain the sections. And there is no need to remove the dye before observation under microscope. - Mount a coverglass on the stained section.
Note: No special mounting reagent is needed. The rest of the dye works as the mounting reagent. - Examine the section under a normal light microscope.
- Fix the wooden dowel on the sample holder of ultramicrotome to make the resin facing outward.
Recipes
- FAA solution
1 ml formalin
1 ml acetic acid
9 ml 99.5% ethanol
9 ml H2O - 100% ethanol
500 ml ethanol (99.5%, commercially available) approximately 100 g molecular sieves 4A 1/16 - 100% Technovit 7100 (I)
10 ml Technovit 7100
0.1 g Hardener I - 25% Technovit 7100 resin (I)
250 µl 100% Technovit 7100 (I)
750 µl 100% ethanol - 50% Technovit 7100 resin (I)
500 µl 100% Technovit 7100 (I)
500 µl 100% ethanol - 75% Technovit 7100 resin (I)
750 µl 100% Technovit 7100 (I)
250 µl 100% ethanol - 100% Technovit 7100 (II)
1 ml Technovit 7100 (I)
66 µl Hardener II
Acknowledgments
This work was funded by the Ministry of Education, Culture, Sports, Science and Technology Grant-in-Aid for Scientific Research (no. 23770046). This work was also supported by the following foundation; the Iijima Memorial Foundation for the Promotion of Food Science and Technology, the Japan Prize Foundation, Shorai Foundation for Science and Technology, Wesco Scientific Promotion Foundation, The Towa Foundation for Food Research, the Foundation of Skylark Food Science Institute and Oohara Foundation.
References
- Matsushima, R., Maekawa, M., Fujita, N. and Sakamoto, W. (2010). A rapid, direct observation method to isolate mutants with defects in starch grain morphology in rice. Plant Cell Physiol 51(5): 728-741.
- Matsushima, R., Yamashita, J., Kariyama, S., Enomoto, T., Sakamoto, W. (2013). A phylogenetic re-evaluation of morphological variations of starch grains among Poaceae species. J Applied Glycoscience 60:37-44.
- Matsushima, R., Maekawa, M., Kusano, M., Kondo, H., Fujita, N., Kawagoe, Y. and Sakamoto, W. (2014). Amyloplast-localized SUBSTANDARD STARCH GRAIN4 protein influences the size of starch grains in rice endosperm. Plant Physiol 164(2): 623-636.
Article Information
Copyright
© 2014 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Matsushima, R. (2014). Thin Sections of Technovit 7100 Resin of Rice Endosperm and Staining. Bio-protocol 4(18): e1239. DOI: 10.21769/BioProtoc.1239.
-
Matsushima, R., Maekawa, M., Kusano, M., Kondo, H., Fujita, N., Kawagoe, Y. and Sakamoto, W. (2014). Amyloplast-localized SUBSTANDARD STARCH GRAIN4 protein influences the size of starch grains in rice endosperm. Plant Physiol 164(2): 623-636.
Category
Plant Science > Plant cell biology > Tissue analysis
Plant Science > Plant cell biology > Cell imaging
Biochemistry > Carbohydrate > Polysaccharide
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Tips for asking effective questions
+ Description
Write a detailed description. Include all information that will help others answer your question including experimental processes, conditions, and relevant images.
Share
Bluesky
X
Copy link