- Submit a Protocol
- Receive Our Alerts
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Trichome Isolation and Integrity Test from Brassica villosa and Other Species
Published: Vol 4, Iss 24, Dec 20, 2014 DOI: 10.21769/BioProtoc.1361 Views: 9404
Reviewed by: Arsalan DaudiCindy AstMaria Sinetova

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols
Tensile Testing Assay for the Measurement of Tissue Stiffness in Arabidopsis Inflorescence Stem
Kouki Yoshida [...] Nobutaka Mitsuda
Aug 5, 2019 8779 Views
Analysis of Monosaccharides from Arabidopsis Seed Mucilage and Whole Seeds Using HPAEC-PAD
Gillian H. Dean [...] George W. Haughn
Dec 20, 2019 4361 Views
Targeting Ultrastructural Events at the Graft Interface of Arabidopsis thaliana by A Correlative Light Electron Microscopy Approach
Clément Chambaud [...] Lysiane Brocard
Jan 20, 2023 871 Views
Abstract
The outward growths from one or more epidermal cells are well known as trichomes (plant hair cells) (Levin, 1973; Mathur, 2006). Preparation of pure intact non-glandular trichomes from trichome-rich Brassica villosa depended on rendering the trichomes sufficiently stiff to dislodge them from the leaves in an undamaged state, which can be further used for structural, transcriptome, genome and biochemical or chemical analysis. Dislodging the flexible trichomes from Brassica villosa (B. villosa) with a paintbrush [as in Zhang and Oppenheimer (2004)] proved too gentle, and scraping trichomes off the leaves with a razor blade [as in Zhang and Oppenheimer (2004)] resulted in trichome cell disruption. Aziz et al. (2005) reported isolating alfalfa glandular trichomes by shearing in liquid nitrogen (N2). In the present study, a similar method was used. Non-glandular leaf trichomes were isolated by treating tissue with liquid N2 to stiffen the flexible trichomes, followed by 1 min of shearing force with a common vortex mixer to dislodge the trichomes from their leaf bed (Nayidu et al., 2014). Up-to ~20 % of the trichomes were removed from the leaf surface and the majority were unbroken (confirmed by no staining by trypan blue; Figure 1). Trichomes were then purified by straining a tissue/trichome/water mixture through a sieve. However, leaf tissues also became very brittle after dipping in liquid N2 and broke up into very small pieces if the leaf tissue was agitated for a longer time period. Hence, N2-treated leaf samples could not be re-used to recover remaining attached trichomes. Contaminating leaf pieces from a 1 min shear were larger and easily to separate manually from the detached trichomes within the sieve, rendering a purified intact trichome preparation. The method was also successful at purifying trichomes from soybean (Glycine max) and tomato (Solanum lycopersicum).
Keywords: TrichomeMaterials and Reagents
- Fresh trichome-bearing leaf tissue from Brassica villosa (drepanensis cv.) (from two month old up-to two year leaves), soybean cv. B220, and tomato cv. Roma
Note: Depending on the plant species and the subsequent type of analysis after trichome isolation, any tissue with non-glandular trichomes will likely suffice. However, tissues with denser trichome coverage will provide a greater yield of detached trichomes. - Liquid nitrogen
Note: Training in the safe use of liquid nitrogen is imperative. - Sterile Millipore purified water
- Ice
- 0.4 % trypan blue (see Recipes)
Equipment
- Gloves to resist freezing temperatures (Cryo-Gloves) (VWR International, catalog number: 32885-736 )
- Polyfoam insulated box with ice
- 1-ml pipette with plastic pipette tips
- 50 ml BD FalconTM conical plastic centrifuge tubes (BD, catalog number: 14-432-22 ) or plastic 250 ml centrifuge bottles (Thermo Fisher Scientific, catalog number: EW-06105-20 )
- BD-Falcon nylon mesh cell strainer (40 μm pore size) (BD Biosciences, catalog number: 08-771-1 )
- Blunt end forceps to remove contaminating debris from the strainer (Seton, catalog number: AA969 )
- Genie 2 vortex mixer (Thermo Fisher Scientific) having round mixer head (for tubes) and flat mixer head (for larger bottles)
- -80 °C freezer
- 4 °C refrigerator
- FlexidryTM unit (FTS Systems, catalog number: 4283 )
- Controlled environment greenhouse or growth cabinet.
Note: Plant growth conditions depend on the nature of subsequent analyses. For example, chemical composition of plant tissuses can change substantially with altered light intensity and temperature (16 h light/8 h dark, at least 400 µE.m-2.s-1, 20/17 °C recommended, but light intensity from 700-1,400 µE.m-2.s-1 will mimic outside daylight). - Axiovert 100 microscope (ZEISS) with 10x magnification
Procedure
- Healthy trichome-bearing leaves of B. villosa, soybean, and tomato were selected after growth in a greenhouse.
Note: For B. villosa, up to 2 year old plant leaves can be used. - Harvested leaves were cut into different sizes based on the size of the vessel used for the isolation of trichome (see Video 1 below).
Note: One 3 cm x 3 cm leaf piece if using a 50 ml tube and two 10 cm x 4 cm strips if using 250 ml centrifuge bottles. The choice of tube and tissue size is dependent on the amount of trichome tissue needed for subsequent analyses (see recommendations below). For any leaf size used, the maximum purity trichome preparation achieved is ~20% of the total trichomes present on the leaf piece. - Maximum 30% of the tube or bottle was filled with liquid N2 and capped (loosely screwed on) to prevent overflow during mixing (without tube breakage from N2 vapour pressure).
- Using gloves, tubes were mixed for 1 min in liquid N2 using a Genie 2 vortex mixer at maximum speed (level 8). One min vortexing maximizes the recovery of detached trichomes while minimizing the disruption of leaf tissue into small contaminating fragments that are difficult to remove later.
- The tubes were then kept fully open on ice to maximize liquid N2 evaporation.
- Large leaf pieces were manually removed from the tubes using forceps and retained as a trichome-stripped leaf control sample.
- Released trichomes adhering to the inside walls of the tube were gently suspended by swirling in 2 ml of sterile Millipore purified water.
- Water initially frozen in the tube (because of liquid N2) was kept on ice for 3-4 min to thaw.
- Thawed water from the tubes having trichomes (and also minute pieces of leaves) was sieved through a BD-Falcon nylon mesh cell strainer into a fresh 50 ml tubes.
- Minute pieces of leaves on the cell strainer netting were again manually removed using forceps.
- The strainer was then inverted over the same fresh tube.
- Trichomes were dislodged into the tube by gently sweeping them (i.e. rinsing the strainer with a pipette) using an additional 1-2 ml of Millipore water (see Video 1 below).
Note: Cell strainers could be used up to 8-10 times before the pores became clogged. Intensive careful cleaning will help lengthen strainer use for additional 2-3 times, but new ones are preferred after pore clogging, and further cleaning will result in a hole in the strainer. - Freshly prepared trichome batches (in water) and trichome-dislodged leaves (control) were immediately stored at 4 °C separately until all tissue sections were processed.
- 1 ml of water containing trichomes was stained fresh by adding 0.5 ml of 0.4 % trypan blue. Contents were incubated for 3-4 h at room temperature (in the light or dark) without shaking in a petri-dish in preparation for microscopy.
- An Axiovert 100 microscope (10x magnification) was used to confirm the proportion of un-ruptured trichome cells and the quality of the preparation.
Notes:- The trichome isolation protocol has no washing step, but to obtain the image of trichomes (Figure 1) we conducted a washing step using water for improving the appearance of the trichomes.
- Intact cells are unstained; disrupted cells are stained blue; a poor quality preparation would contain more disrupted trichomes and/or a greater proportion of contaminating leaf tissue fragments compared with Figure 1.
- The trichome isolation protocol has no washing step, but to obtain the image of trichomes (Figure 1) we conducted a washing step using water for improving the appearance of the trichomes.
- The samples were then flash frozen in liquid N2, stored at -80 °C for 4-5 h, and freeze dried overnight in a FlexidryTM unit.
- The method can be used for purifying non-glandular trichomes from other plant species (Figure 1).
- Suggested leaf sample size for isolating B. villosa trichomes for various purposes (leaves with 300-500 µm trichome length, ~4,000 trichomes/cm2 and 1 mg of dried trichomes isolation from 100 g of fresh leaf tissue [FL]): (a) for electron microscopic structural analysis (100/cm2); (b) for RNA isolation (300 g FL); (c) for biochemical extraction (500 g FL); for HPLC-UV analysis (500 g FL); for NMR structural analysis (800-1,000 g FL); for metal content analysis by inductively coupled plasma mass spectroscopy (500 g FL).
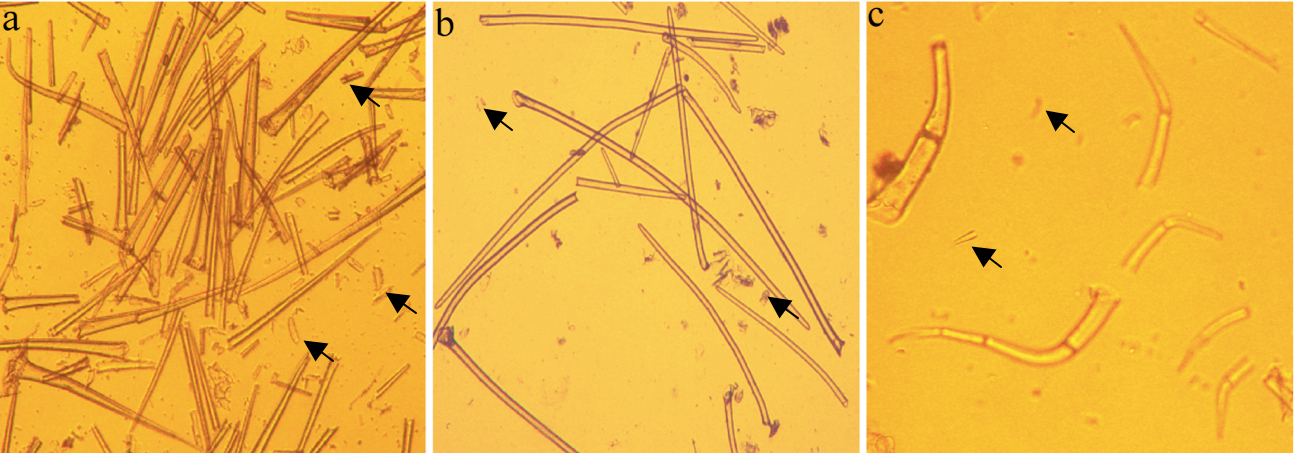
Figure 1. High quality trichome preparations at 10x magnification after isolation from a Brassica villosa b Soybean (Glycine max) c Tomato (Solanum lycopersicum). Arrows indicate contaminating leaf tissue fragments or broken trichomes.
Representative data
Recipes
- 0.4 % trypan blue
40 mg of trypan blue dissolved in 100 ml Millipore-filtered water
Acknowledgments
This research was supported by grants from the Canola Council of Canada, Sask-Canola, the Saskatchewan Agriculture Development Fund, and funding from Agriculture and Agri-Food Canada.
References
- Aziz, N., Paiva, N. L., May, G. D. and Dixon, R. A. (2005). Transcriptome analysis of alfalfa glandular trichomes. Planta 221(1): 28-38.
- Koona, P. and Jackai, L. (2004). The potential of pod-shaving in studies of the role of trichomes in Vigna resistance to the pod-bug Clavigralla tomentosicollis Stål (Hemiptera: Coreidae). International Journal of Tropical Insect Science 24(04): 298-303.
- Levin, D. A. (1973). The role of trichomes in plant defense. Q Rev Biol: 3-15.
- Mathur, J. (2006). Trichome cell morphogenesis in Arabidopsis: a continuum of cellular decisions. This review is one of a selection of papers published in the Special Issue on Plant Cell Biology. Botany 84:604-612.
- Nayidu, N. K., Tan, Y., Taheri, A., Li, X., Bjorndahl, T. C., Nowak, J., Wishart, D. S., Hegedus, D. and Gruber, M. Y. (2014). Brassica villosa, a system for studying non-glandular trichomes and genes in the Brassicas. Plant Mol Biol 85(4-5): 519-539.
- Zhang, X. and Oppenheimer, D. G. (2004). A simple and efficient method for isolating trichomes for downstream analyses. Plant Cell Physiol 45(2): 221-224.
Article Information
Copyright
© 2014 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Nayidu, N., Bonham-Smith, P. and Gruber, M. Y. (2014). Trichome Isolation and Integrity Test from Brassica villosa and Other Species. Bio-protocol 4(24): e1361. DOI: 10.21769/BioProtoc.1361.
Category
Plant Science > Plant cell biology > Tissue analysis
Plant Science > Plant physiology > Tissue analysis
Cell Biology > Tissue analysis > Tissue isolation
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Tips for asking effective questions
+ Description
Write a detailed description. Include all information that will help others answer your question including experimental processes, conditions, and relevant images.
Share
Bluesky
X
Copy link








