- Submit a Protocol
- Receive Our Alerts
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Cartilage Induction from Mouse Mesenchymal Stem Cells in High-density Micromass Culture
Published: Vol 9, Iss 1, Jan 5, 2019 DOI: 10.21769/BioProtoc.3133 Views: 5796
Reviewed by: Giusy TornilloSurabhi SonamJaira Ferreira de Vasconcellos

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols
Protocol for Isolation of Cardiomyocyte from Adult Mouse and Rat
Huiliang Zhang and Peter S. Rabinovitch
May 20, 2022 2282 Views
A Step-by-step Protocol for Obtaining Mature Microglia from Mice
Min-Jung Yoo and Min-Soo Kwon
Aug 5, 2022 1795 Views
Establishment of an in vitro Differentiation and Dedifferentiation System of Rat Schwann Cells
Ying Zou
Mar 5, 2023 415 Views
Abstract
Mesenchymal stem cells have the ability to differentiate into multiple lineages, including adipocytes, osteoblasts and chondrocytes. Mesenchymal stem cells can be induced to differentiate into chondrocytes in extracellular matrices, such as alginate or collagen gel. Mesenchymal stem cells in a cell pellet or micromass culture can be also induced to form cartilages in a defined medium containing chondrogenic cytokines, such as transforming growth factor-β (TGF-β). Here, we describe a simple method to form cartilage by seeding mesenchymal cells derived from limb-bud cells at high cell density. First, we dissected the limb buds from embryonic mice (embryonic day 12.5) and digested them with enzymes (dispase and collagenase). After filtration using a cell strainer, we seeded the cells at high density. Unlike other methods, the method described here is simple and does not require the use of specialized equipment, expensive materials or complex reagents.
Keywords: ChondrocytesBackground
Mesenchymal cells differentiate into skeletal elements by forming a cartilaginous nodule, which induces bone formation through endochondral ossification in the vertebral column and long bones (Karsenty et al., 2009). Endochondral ossification is required for proper skeletal development during embryogenesis and has been recently demonstrated to be involved in the bone regeneration and joint diseases postnatally (Kawaguchi, 2008). Mesenchymal cells could be used as a regenerative therapy for these diseases. To study the mechanisms regulating endochondral skeletal development, we have examined high-density micromass cultures of embryonic limb-bud mesenchymal cells (Gay and Kosher, 1984; Iezaki et al., 2018). The in vitro chondrogenic cell culture system is useful for the analysis cartilage nodule formation that results from the condensation of mesenchymal cells and differentiation into chondrocytes.
Several methods have been developed to generate cartilage from mesenchymal cells. Mesenchymal cells in cell pellet or micromass culture can be induced to form cartilage in chondrogenic medium containing cytokines such as transforming growth factor-β (TGF-β) (Sekiya et al., 2002). Mesenchymal cells have also been induced to differentiate into chondrocytes in extracellular matrices such as alginate or collagen gel (Xu et al., 2008). Although these methods are effective to analyze chondrogenesis, they can be time consuming and expensive because they require complex reagents and equipment.
For this reason, we have developed a culture method to form cartilage from mesenchymal cells using a very simple method. We induced the differentiation of cells harvested from mouse limb buds (embryonic day 12.5) in high-density micromass culture and induced the cells to form cartilage nodules in growth medium without chondrogenic cytokines. In comparison to other methods, the method described here is simple and does not require specialized equipment, expensive materials or complex reagents. This method could be really useful for reducing the cost and complexity of the procedure.
Materials and Reagents
- Pipette tips (RIKAKEN, catalog numbers: RST 481SCV, RST 4820YV, RST 4810BV)
- 1.5 ml tube (WATSON, catalog number: 131-815C)
- Cell strainer (Corning, catalog number: 352235)
- 4-well cell culture plate (Thermo Fisher Scientific, catalog number: 176740)
- Syringe-driven filter unit (Millipore, catalog number: SLGV033RS)
- Filter paper (ADVANTEC, catalog number: 00011090)
- E12.5 Embryo mice (C57BL/6J)
- D-MEM/Ham's F-12 (Wako, catalog number: 048-29785)
- Dispase (Gibco, catalog number: 17105-041)
- Collagenase (Wako, catalog number: 034-22363)
- FBS (Sigma, NRE-172012)
- L(+)-Ascorbic Acid (Wako, catalog number: 016-04805)
- Paraformaldehyde (PFA) (Wako, catalog number: 162-16065)
- Sodium Hydroxide (Wako, catalog number: 198-13765)
- Acetic acid (Wako, catalog number: 017-00251)
- 10x PBS(-) (Wako, catalog number: 314-90185)
- Alcian Blue 8GX powder (Sigma, catalog number: A5268-25G)
- Enzyme solution (see Recipes)
- L(+)-Ascorbic acid stock solution (see Recipes)
- Chondrogenic medium (see Recipes)
- 8% PFA stock solution (see Recipes)
- 1% acetic acid solution (see Recipes)
- Alcian blue solution (see Recipes)
Equipment
- Pipettes (Glison, catalog numbers: F144802, F123615, F123602)
- Microtube rotator (AS ONE, catalog number: MTR-103)
- CO2 incubator (SANYO, model: MCO-17AIC)
- High speed refrigerated microcentrifuge (TOMY, model: MX-307)
- Automated cell counter (Bio-Rad, model: TC-20)
- Microscope (Kyence, model: BZ-9000)
Software
- BZ-2 application (Keyence)
- ImageJ (NIH, https://imagej.nih.gov/ij/)
Procedure
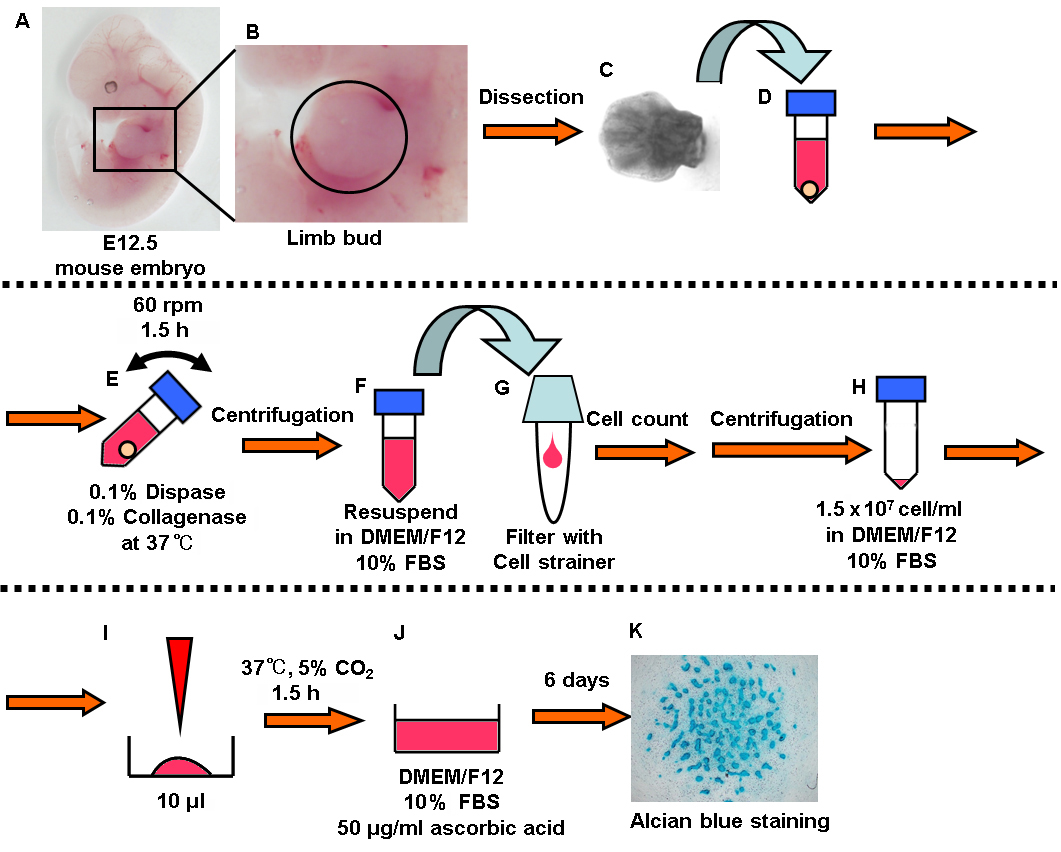
Figure 1. Summary of experimental procedure. In brief: A-C. Dissect the limb buds from embryonic mice. D-E. Digested them with enzymes (dispase and collagenase). F-I. Seed the cells at high density. J. Add the chondrogenic medium and incubate for 6 days. K. Alcian blue staining.
- Preparation of the cells
- Dissect the limb buds from mouse embryonic day 12.5 (the part surrounded by circle in Figure 1B). One limb bud is enough to induce 2 or 3 wells of cartilage (Figures 1A-1C).
- Collect the limb buds in a 1.5 ml tube and add 1 ml enzyme solution (0.1% Dispase, 0.1% Collagenase in D-MEM/Ham's F-12) (Figure 1D).
- Rotate the tube at 60 rpm on a microtube rotator (AS ONE) for 1.5 h at 37 °C (Figure 1E).
- Centrifuge the tube for 5 min at 300 x g and resuspend the cells in 1 ml D-MEM/Ham's F-12 (Figure 1F).
- Apply the cell suspension to a 40 μm cell strainer placed in a 5 ml round bottom tube and measure the cell density using an automated cell counter (Bio-Rad) (Figure 1G).
- Cartilage formation
- Centrifuge the tube for 5 min at 300 x g and resuspend the cells in D-MEM/Ham's F-12 containing 10% FBS to adjust the cell density to 1.5 x 107 cells/ml (Figure 1H).
- Place a 10 μl droplet in 4-well culture plate and incubate the cells in the CO2 incubator for 1.5 h (Figure 1I).
- Add the 500 μl chondrogenic medium (D-MEM/Ham's F-12 containing 10% FBS and 50 μg/ml L-ascorbic acid) with care not to disrupt the droplet and incubate in the CO2 incubator (Figure 1J).
- Change the medium every 2 days and incubate for 6 days.
- Alcian blue staining (Figure 1K)
- Fix the cells in 500 μl 4% PFA for 10 min and wash the cells twice using Milli-Q water.
- Add 500 μl 1% acetic acid for 5 min and replace acetic acid by 500 μl Alcian blue solution for 30 min.
- Wash the cells with 500 μl 1% acetic acid and wash twice using Milli-Q water.
- Image the stained cells using optical microscope (Figure 2).
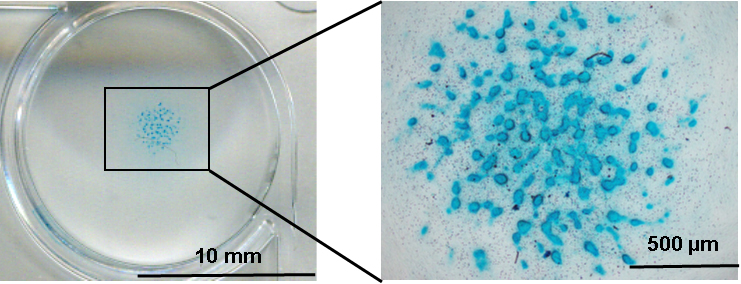
Figure 2. Alcian blue staining
Data analysis
Alcian blue stained areas were measured with ImageJ software (Abramoff et al., 2004). Appropriate statistical analysis should be carried out on the obtained data.
Recipes
- Enzyme solution
Dissolve the dispase (Gibco) and collagenase (Wako) (0.1% w/v each) in D-MEM/Ham's F-12 (Wako) and filter with a 0.22 μm syringe filter (Millipore) - L(+)-Ascorbic acid stock solution
Dissolve the ascorbic acid (Wako) (50 mg/ml) in PBS(-) and filter with syringe filter (Millipore) - Chondrogenic medium
D-MEM/Ham's F-12 (Wako) containing 10% FBS (Sigma) and 50 μg/ml L-ascorbic acid - 8% PFA stock solution
Add the 40 g of PFA (Wako) in 400 ml of hot Milli-Q water (60 °C) and add 50 μl of 10 N NaOH (Wako), continue to stir until PFA dissolves
Add 50 ml of 10x PBS(-) and adjust to final volume of 500 ml - 1% acetic acid solution
Add 1 ml of acetic acid (Wako) to 99 ml of Milli-Q water - Alcian blue solution
Dissolve 1 g of Alcian blue 8GX (Sigma) in 100 ml of 3% acetic acid solution (Wako) and filter with filter paper (ADVANTEC)
Acknowledgments
This work was supported in part by the Japan Society for the Promotion of Science (16H05131 and 17KT0051 to E.H.), the Japan Agency for Medical Research and Development (17824969 to E.H.).
Competing interests
The authors declare no conflict of interest.
Ethics
The study protocol meets the guidelines of the Japanese Pharmacological Society and was approved by the Committee for Ethical Use of Experimental Animals at Kanazawa University, Kanazawa, Japan.
References
- Abramoff, M. D., Magalhães, P. J. and Ram, S. J. (2004). Image processing with ImageJ. Biophotonics International 11(7): 36-42.
- Gay, S. W. and Kosher, R. A. (1984). Uniform cartilage differentiation in micromass cultures prepared from a relatively homogeneous population of chondrogenic progenitor cells of the chick limb bud: effect of prostaglandins. J Exp Zool 232(2): 317-326.
- Iezaki, T., Horie, T., Fukasawa, K., Kitabatake, M., Nakamura, Y., Park, G., Onishi, Y., Ozaki, K., Kanayama, T., Hiraiwa, M., Kitaguchi, Y., Kaneda, K., Manabe, T., Ishigaki, Y., Ohno, M. and Hinoi, E. (2018). Translational control of Sox9 RNA by mTORC1 contributes to skeletogenesis. Stem Cell Reports 11(1): 228-241.
- Karsenty, G., Kronenberg, H. M. and Settembre, C. (2009). Genetic control of bone formation. Annu Rev Cell Dev Biol 25: 629-648.
- Kawaguchi, H. (2008). Endochondral ossification signals in cartilage degradation during osteoarthritis progression in experimental mouse models. Mol Cells 25(1): 1-6.
- Sekiya, I., Vuoristo, J. T., Larson, B. L. and Prockop, D. J. (2002). In vitro cartilage formation by human adult stem cells from bone marrow stroma defines the sequence of cellular and molecular events during chondrogenesis. Proc Natl Acad Sci U S A 99(7): 4397-4402.
- Xu, J., Wang, W., Ludeman, M., Cheng, K., Hayami, T., Lotz, J. C. and Kapila, S. (2008). Chondrogenic differentiation of human mesenchymal stem cells in three-dimensional alginate gels. Tissue Eng Part A 14(5): 667-680.
Article Information
Publication history
Accepted: Dec 12, 2018
Published: Jan 5, 2019
Copyright
© 2019 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Iezaki, T., Fukasawa, K., Yamada, T., Hiraiwa, M., Kaneda, K. and Hinoi, E. (2019). Cartilage Induction from Mouse Mesenchymal Stem Cells in High-density Micromass Culture. Bio-protocol 9(1): e3133. DOI: 10.21769/BioProtoc.3133.
Category
Developmental Biology > Cell signaling > Fate determination
Cell Biology > Cell isolation and culture > Cell differentiation
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Tips for asking effective questions
+ Description
Write a detailed description. Include all information that will help others answer your question including experimental processes, conditions, and relevant images.
Share
Bluesky
X
Copy link