- Submit a Protocol
- Receive Our Alerts
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
A New Efficient Method for Measuring Oxygen Consumption Rate Directly ex vivo in Human Epidermal Biopsies
(*contributed equally to this work) Published: Vol 9, Iss 5, Mar 5, 2019 DOI: 10.21769/BioProtoc.3185 Views: 5795
Reviewed by: mohan babuAmriti Rajender LullaAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols
Surgical Induction of Endometriosis in Female Mice
Alejandra Escudero-Lara [...] Rafael Maldonado
Sep 20, 2020 2992 Views
Preparing Viable Hippocampal Slices from Adult Mice for the Study of Sharp Wave-ripples
Linhua Liu [...] Jian-young Wu
Oct 5, 2020 3068 Views
Intravenous and Non-invasive Drug Delivery to the Mouse Basal Forebrain Using MRI-guided Focused Ultrasound
Kristiana Xhima [...] Isabelle Aubert
Jun 20, 2021 3311 Views
Abstract
Skin cells are constantly exposed to environmental influences such as air pollution, chemicals, pathogens and UV radiation. UV radiation can damage different biological structures, but most importantly cellular DNA. Mitochondria contain their own genome and accumulate UV-induced DNA mutations to a large extent. This can result, e.g., in accelerated skin aging. Understanding the impact of harmful external influences on mitochondrial function is therefore essential for a better view on the development of age-related diseases. Previous studies have been carried out on cell cultures derived from primary cells, which does not fully represent the real situation in the skin, while the mitochondrial parameters were considered barely or not at all. Here we describe a method to measure mitochondrial respiratory parameters in epithelial tissue derived from human skin biopsies using an Agilent Seahorse XF24 Flux Analyzer. Before the assay, epidermis and dermis are separated enzymatically, we then used the XF24 Islet capture microplates to position the epidermis samples to measure oxygen consumption rates (OCR) and extracellular acidification rates (ECAR). In these plates, small nets can be fixed to the plate bottom. The epidermis was placed with the vital–basal–side on the net. Active ingredients in the three ports were injected consecutively to determine the effect of each compound. This allows determining the efficiency of the individual complexes within the respiratory chain. This protocol enables the testing of toxic substances and their influence on the mitochondrial respiration parameters in human epithelial tissue.
Keywords: Oxygen consumption rateBackground
The human skin is the largest organ of the human body and functions as a physical barrier shielding the body from a number of harmful external agents such as air pollution or solar radiation (Gebhard et al., 2014). Solar radiation, e.g., induces increased production of reactive oxygen species (ROS) and thereby DNA damage and mutations in skin, which results in skin aging and skin cancer (Gebhard et al., 2014). Mitochondrial dysfunction resulting from DNA damage is thought to play an important role in these and other essential cellular processes. The accumulation of reactive oxygen species (ROS)-damaged mitochondrial DNA (mtDNA) and proteins can induce mitochondrial dysfunction within the electron transport chain (ETC) and in turn lead to an enhanced ROS production and increased mitochondrial dysfunction (Furda et al., 2012; Yoshida et al., 2012). According to the ‘mitochondrial theory of aging’, this vicious cycle is a major cause for cellular aging, tissue dysfunction and degeneration (Harman, 1972). Understanding the impact of harmful external influences on mitochondrial function is therefore essential for a better view on aging in general. Unfortunately, the measurement of mitochondrial respiration in cell cultures cannot completely reflect the real situation in skin. We therefore established an efficient method to measure the mitochondrial respiration ex vivo directly in human epidermis biopsies with the Seahorse XF24 Flux Analyzer and Seahorse XF24 Islet Capture microplates. The XF24 Analyzer is a multi-well plate system which measures oxygen consumption rates (OCR) and extracellular acidification rates (ECAR) by changes in the fluorescence of solid state fluorophores. In this system, up to four different ports can be used to inject compounds to obtain a mitochondrial respiration profile. The procedure described in this protocol includes the preparation of the biopsies, the preparation of the epidermis for insertion into the XF24 Analyzer and the measurement of mitochondrial parameters by the addition of specific mitochondrial inhibitors. This protocol was established for the testing of toxic substances and their influence on the mitochondrial respiration parameters in human epithelial tissue. In addition, age-related effects of various substances can be tested based on the age of the donor. The analysis of mitochondrial respiration in epidermis derived from skin biopsies represents an important addition to existing screenings.
Materials and Reagents
- Centrifuge tubes 15, 50 ml (SARSTEDT, catalog numbers: 62.554.001, 62.547.254)
- Microscope slides (Carl Roth, catalog number: 1879.1)
- Petri dishes (SARSTEDT, catalog number: 83.3902)
- Pipette tips 10 μl, 20 μl, 200 μl, 1,000 μl (SARSTEDT, catalog numbers: 70.1130, 70.116, 70.760.002, 70.762)
- Sterile filter 0.2 μm (SARSTEDT, catalog number: 83.1826.001)
- Human skin biopsies (kept in 1x PBS at 4 °C for a maximum of 36 h)
- 2-Propanol (Carl Roth, catalog number: 9866.1)
- Dispase II (neutral protease, grade II) (Roche, catalog number: 04942078001, storage temp. 4 °C)
- Dulbecco’s modified Eagle’s medium, high glucose (Sigma-Aldrich, catalog number: D7777-10L, storage temp. 4 °C)
- KBMTM Gold Keratinocyte Growth Medium BulletKitTM (Lonza, catalog number: 00192060, storage temp. 4 °C)
- KCl (Carl Roth, catalog number: 6781.3)
- KH2PO4 (Carl Roth, catalog number: 3904.2)
- Na2HPO4·12H2O (Carl Roth, catalog number: N350.1)
- NaCl (Carl Roth, catalog number: 3957.3)
- NaOH (Carl Roth, catalog number: 6771.3)
- Seahorse XF Calibrant Solution (Agilent Technologies, catalog number: 100840-000)
- Seahorse XF Cell Mito Stress Test Kit (Oligomycin, FCCP, Rotenone/Antimycin A) (Agilent Technologies, catalog number: 103015-100, storage temp. -20 °C)
- 10 M NaOH (see Recipes)
- 1x PBS (see Recipes)
- Enzymatic digestion Solution (see Recipes)
- XF assay medium (see Recipes)
Equipment
- Scissor, stainless steel (Carl Roth, catalog number: HCX4.1)
- Seahorse Capture Screen Insert Tool (Agilent Technologies, catalog number: 101135-100)
- Seahorse XF24 Islet Capture FluxPak containing sensor cartridges and Islet Capture Microplates (Agilent Technologies, catalog number: 101174-100)
- Tweezers, stainless steel (Carl Roth, catalog number: 2687.1)
- Incubator at 37 °C without CO2 (GFL, catalog number: 4010)
- pH Meter FiveEasyTM F20 (Mettler Toledo, catalog number: 30266626)
- Pipettes, Eppendorf Research® Plus 10 μl, 20 μl, 200 μl, 1,000 μl (Eppendorf, catalog numbers: 3123000020, 3123000039, 3123000055, 3123000063)
- Seahorse XF24 Flux Analyzer (Agilent Technologies, model: XF24, catalog number: 100737-100)
Software
- Seahorse XF24 Flux Analyzer Software (instrument software) (Agilent Technologies, Version 1.8.1.1)
- Wave (analysis software) (Agilent Technologies, Version 2.6)
Procedure
Day before the assay
- Take a biopsy from the sample tube and wash first in 70% isopropanol followed by 1x PBS to remove any residues of blood and other impurities.
- In the next step, remove fat and tissue (see Figures 1A and 1B).
- Cut the biopsy (see Figure 1B) into small pieces (0.4 x 0.4 cm approximately) (see Figure 1C) and place them in enzymatic digestion solution in a 15 ml centrifuge tube.
- Store the biopsy for enzymatic digestion at 4 °C for 16 h (under non-stirring conditions).
- Hydrate the sensor cartridge in Seahorse XF Calibrant according to the manufacturer’s instructions one day prior to measurement in a non-CO2 incubator.
- Turn on the XF24 analyzer to preheat the system to 37 °C.
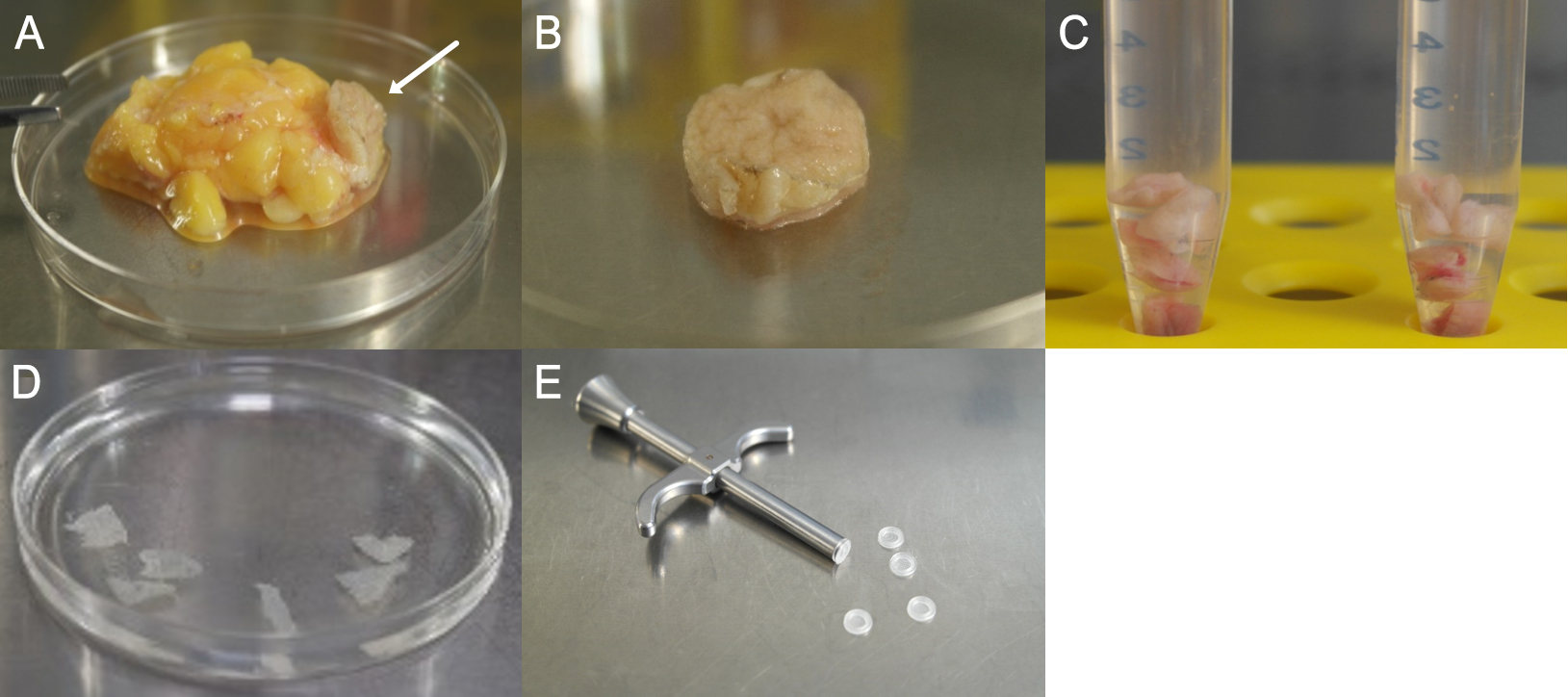
Figure 1. Preparation steps of the biopsy. A. Fat and connective tissue adherent to the dermis/epidermis (arrow). B. Dermis/Epidermis after first purification step. C. Divided biopsy in solution for enzymatic digestion. D. Separated epidermis sheets after incubation with Dispase II. E. Capture screen insert tool with Islet capture screens (Agilent Technologies).
- Prepare the XF assay medium and heat to 37 °C.
- After enzymatic digestion, carefully remove epidermis from the dermis (tweezers) and place in Petri dish with 1x PBS (see Figure 1D). In this step, it is important to ensure that the basal side is facing down towards the bottom of the petri dish. The epidermal sheet should be placed with the vital–basal–side on the capture screen. This orientation is essential for the measurement as the side facing the capture screen gets detected by the sensor (see Figure 2E).
- Carefully lift the epidermis from the 1x PBS with a microscope slide and wipe it off on a second slide (turn over the epidermis once).
- Wipe off the skin with the second slide on the capture screen, pull it smoothly and insert the capture screen with the capture screen insert tool into the Islet capture microplate (see Figure 1E). The epidermis should cover the entire surface of the capture screen to ensure that it’s always to measure the same epidermis area (see Figures 2A-2C). There should be no air bubbles (see Figure 2D) under the epidermis, as they interfere with the measurement.
- Remove excess overhanging skin in the well with tweezers.
- Add 450 μl XF assay medium into each well of the Islet capture microplate.
- Incubate the Islet capture microplate in the incubator (without CO2) at 37 ° C for 45 min to adjust the epidermis to the new medium.
- Load the injection ports of the sensor cartridge as programmed (see the programming of the XF controller), follow the instructions of the XF Analyzer and start the calibration of the sensor cartridge.
- When calibration is complete, place the Islet capture microplate in the XF Analyzer and start measurement.
- Data analysis is performed with the seahorse analysis software Wave.
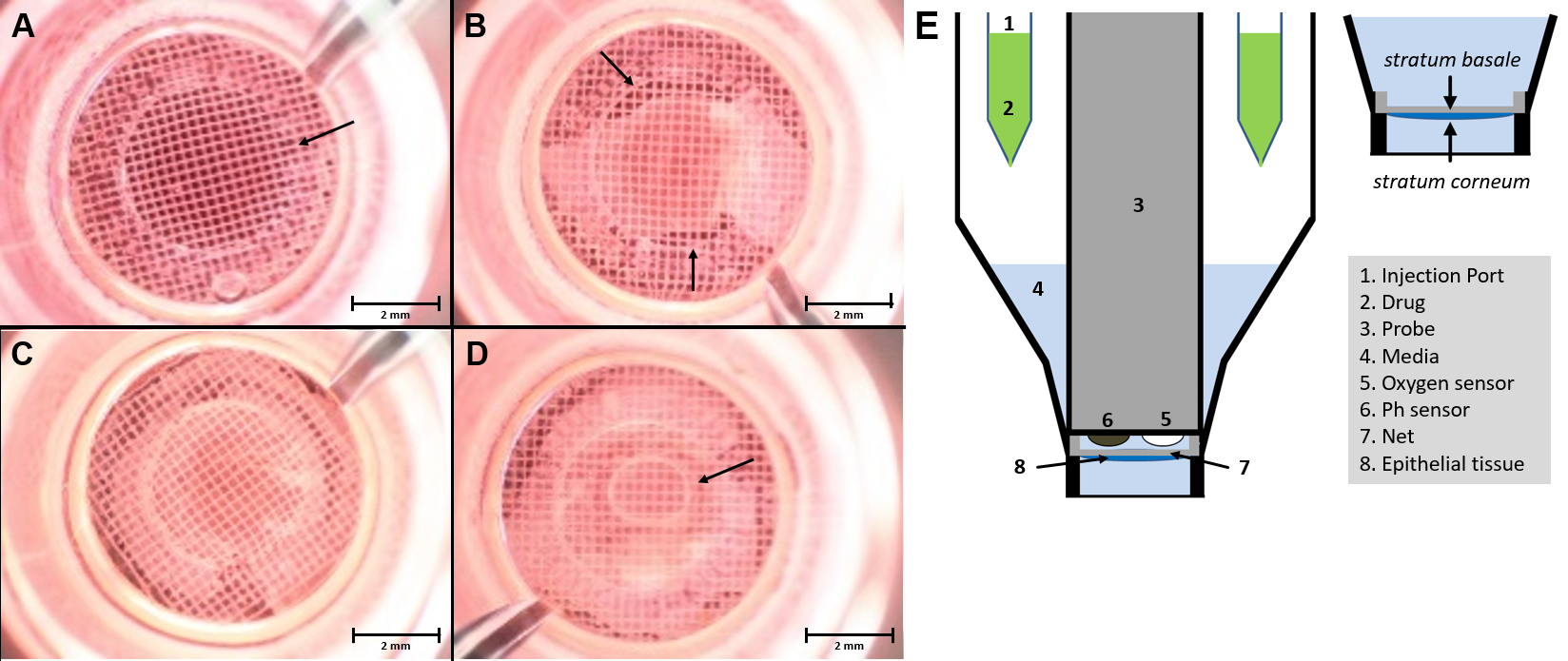
Figure 2. Microscopic image of epidermis in the XF24 islet capture microplate. The epidermis has to be applied as flat as possible with the basal side in contact with the capture screen and then placed in the multiwell plate using the capture screen insert tool. The measurement of oxygen consumption takes place over the surface within the ring marked with the arrow (A). Within the ring in the center of the net, there should be no holes (B) or air bubbles (D). Flat-mounted epidermis, without air bubbles, which covers the entire surface and with the right orientation (C). Schematic setup of the XF24 measuring system with an islet capture microplate (E) (Modified Image from Schniertshauer et al., 2018). Scale bars = 2 mm.
Programming the XF controller
This section describes the individual program steps of the XF controller for measuring the oxygen consumption rate as shown in Figure 2. The stress reagents are diluted to a final concentration of 4 μM with XF assay medium. The concentration of Oligomycin, FCCP and Rotenone/Antimycin A should be determined in a preliminary experiment for the respective experimental conditions. The injection of the stress reagent simultaneously should be carried out in all wells.
Workflow of the XF controller:
- Calibrate Probes
- Time of Delay: 15 min
- First Loop–3 times (Basal state)
- Mixing: 4 min
- Time of Delay: 2 min
- Measurement: 3 min
- Injection of Port A (50 μl Oligomycin, 4 μM final concentration)
- Use oligomycin to block the ATP synthase.
- Then equivalent the decrease in OCR to the amount of oxygen used for the synthesis of ATP.
- Second Loop–6 times (decrease of ATP-linked respiration)
- Mixing: 4 min
- Time of Delay: 2 min
- Measurement: 3 min
- Injection of Port B (55 μl FCCP, 4 μM final concentration)
The decoupling of the respiratory chain by FCCP allows the maximal respiration and the spare respiratory capacity to be determined independently of the proton gradient. - Third Loop–3 times (potentially uncoupled respiration)
- Mixing: 4 min
- Time of Delay: 2 min
- Measurement: 3 min
- Injection of Port C (60 μl Rotenone/Antimycin A, 4 μM final concentration)
In the last Loop, complexes I and II are blocked by the addition of Rotenone and Antimycin A. The remaining oxygen consumption thus depends only on the non-mitochondrial respiration. - Fourth Loop–6 times (non-mitochondrial respiration)
- Mixing: 4 min
- Time of Delay: 2 min
- Measurement: 3 min
- End of Program
Data analysis
Data can be analyzed using Prism 7.04 (GraphPad Software, Inc.). Values are presented as mean ± SEM, or individual values. Five replicates per experimental group consisting of one biopsy are recommended.
Representative data
Measurement of oxygen consumption rate in epidermal tissue derived from human skin biopsies according to the proceeding steps shows the typical course after addition of the four stress reagents Oligomycin, FCCP, Rotenone/Antimycin A as depicted in Figure 3.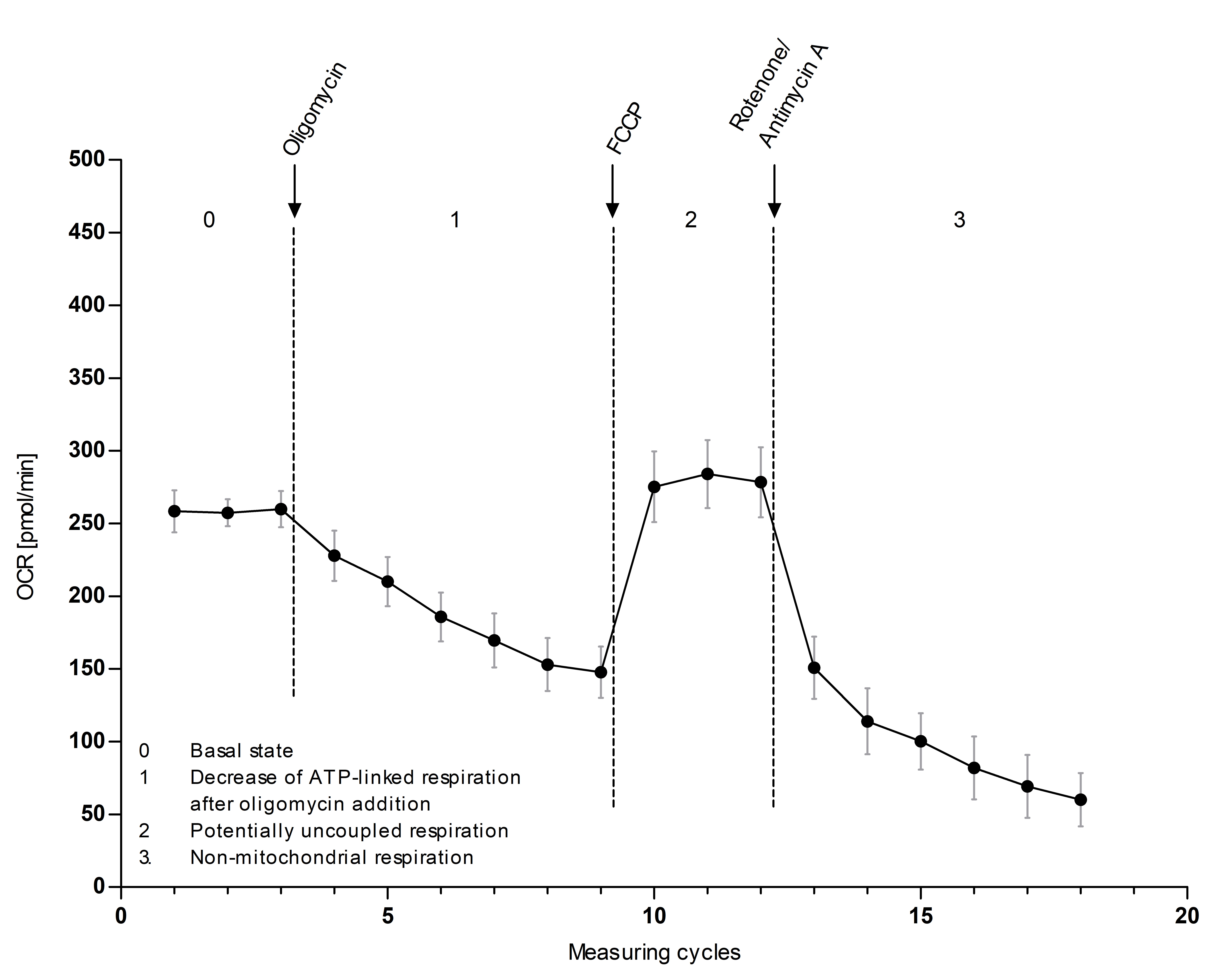
Figure 3. Oxygen consumption rate (OCR) in epithelial tissue derived from human epidermal biopsies. Scheme depicting the main stages after injecting the four active ingredients in three injecting steps. This makes it possible to determine the efficiency of the individual complexes within the respiratory chain (n = 5, mean ± SEM).
Notes
- The measurement of oxygen consumption rates in epithelial tissue derived from human epidermal biopsies we described here was highly reproducible.
- After surgery, biopsies should be kept in PBS at 4 °C for a maximum of 36 h. It is important to carry out the OCR measurement in this time frame as the respiratory parameters decrease significantly after this period.
- The concentration of Oligomycin, FCCP and Rotenone/Antimycin A should be determined in a preliminary experiment for the respective experimental conditions. The indicated concentrations may differ for other biopsies. In preliminary studies, the concentration of 4 μM was determined. Lower concentrations show, in contrast to monolayer cell cultures, no or little effects.
- Because a biopsy is a cell complex in which the substances simply take longer to penetrate the entire sample, a higher concentration and more measurement point as stated in the Seahorse manual must be used.
- In order to exclude photoaging-effect, only biopsies obtained from the same body region should be compared. Samples from body regions with a high UV exposure (e.g., eyelids) show a significantly lower mitochondrial respiration on average than samples with a low UV exposure (e.g., abdomen) from donors with the same age.
Recipes
- 10 M NaOH
40 g NaOH
ad 100 ml ddH2O - 1x PBS
8 g NaCl
0.20 g KCl
2.88 g Na2HPO4·12H2O
1.24 g KH2PO4
ad 1 L ddH2O, pH 7.4 - Enzymatic digestion solution
8 ml KBMTM Gold Keratinocyte Growth Medium BulletKitTM
4 ml Dispase II
Final concentration 2.4 U/ml - XF assay medium
0.675 g Dulbecco’s Modified Eagle’s Medium-high glucose
ad 50 ml ddH2O, sterile filtering, pH 7.4
Acknowledgments
The authors thank Dr. Hug, Dr. Huth, Dr. Astfalk and Dr. Then-Schlagau for the kind supply of samples. They also thank Dr. Franz Enzmann and Dr. Alexander Bürkle for scientific advice. This work was supported with funds from the Baden-Württemberg Ministry of Science, Research and Art and the BMBF–FHprofUnt2012 “MitoFunk” [03FH022PX2].
Competing interests
One of the authors has consulting contracts with MSE Pharmazeutika GmbH, Bad Homburg, Germany and Beiersdorf AG, Hamburg, Germany.
Ethics
All experiments were conducted in accordance with the Declaration of Helsinki and approved by the Ethics Commission of the State Medical Association of Baden-Württemberg, Germany (187-03). Patients were informed in advance and gave their written consent to the use of their samples.
References
- Furda, A. M., Marrangoni, A. M., Lokshin, A. and Van Houten, B. (2012). Oxidants and not alkylating agents induce rapid mtDNA loss and mitochondrial dysfunction. DNA Repair (Amst) 11(8): 684-692.
- Gebhard, D., Matt, K., Burger, K. and Bergemann, J. (2014). Shortwave UV-induced damage as part of the solar damage spectrum is not a major contributor to mitochondrial dysfunction. J Biochem Mol Toxicol 28(6): 256-262.
- Harman, D. (1972). The biologic clock: the mitochondria? J Am Geriatr Soc 20(4): 145-147.
- Schniertshauer, D., Gebhard, D. and Bergemann, J. (2018). Age-dependent loss of mitochondrial function in epithelial tissue can be reversed by coenzyme Q10. J Aging Res 2018: 6354680.
- Yoshida, T., Goto, S., Kawakatsu, M., Urata, Y. and Li, T. S. (2012). Mitochondrial dysfunction, a probable cause of persistent oxidative stress after exposure to ionizing radiation. Free Radic Res 46(2): 147-153.
Article Information
Publication history
Accepted: Feb 14, 2019
Published: Mar 5, 2019
Copyright
© 2019 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Schniertshauer, D., Gebhard, D. and Bergemann, J. (2019). A New Efficient Method for Measuring Oxygen Consumption Rate Directly ex vivo in Human Epidermal Biopsies. Bio-protocol 9(5): e3185. DOI: 10.21769/BioProtoc.3185.
Category
Cell Biology > Tissue analysis > Physiology
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Tips for asking effective questions
+ Description
Write a detailed description. Include all information that will help others answer your question including experimental processes, conditions, and relevant images.
Share
Bluesky
X
Copy link







