- Submit a Protocol
- Receive Our Alerts
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
An Operant Conditioning Model Combined with a Chemogenetic Approach to Study the Neurobiology of Food Addiction in Mice
Published: Vol 10, Iss 19, Oct 5, 2020 DOI: 10.21769/BioProtoc.3777 Views: 4041
Reviewed by: Arnau Busquets-GarciaGian Marco LeggioMarco Venniro

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols
A Simplified Paradigm of Late Gestation Transient Prenatal Hypoxia to Investigate Functional and Structural Outcomes from a Developmental Hypoxic Insult
Elyse C. Gadra and Ana G. Cristancho
Oct 5, 2022 674 Views

Conditioned Lick Suppression: Assessing Contextual, Cued, and Context-cue Compound Fear Responses Independently of Locomotor Activity in Mice
Youcef Bouchekioua [...] Yu Ohmura
Dec 5, 2022 407 Views
In situ Microinflammation Detection Using Gold Nanoclusters and a Tissue-clearing Method
Fayrouz Naim [...] Masaaki Murakami
Apr 5, 2023 702 Views
Abstract
The study of food addiction comprises 3 hallmarks that include the persistence to response without an outcome, the strong motivation for palatable food, and the loss of inhibitory control over food intake that leads to compulsive behavior in addicted individuals. The complex multifactorial nature of this disorder and the unknown neurobiological mechanistic correlation explains the lack of effective treatments. Our operant conditioning model allows deciphering why some individuals are vulnerable and develop food addiction while others are resilient and do not. It is a translational approach since it is based on the Diagnostic and Statistical Manual of Mental Disorders 5th edition (DSM-5) and the Yale Food Addiction Scale (YFAS 2.0). This model allows to evaluate the addiction criteria in 2 time-points at an early and a late period by grouping them into 1) persistence to response during a period of non-availability of food, 2) motivation for food with a progressive ratio, and 3) compulsivity when the reward is associated with a punishment such as an electric foot-shock. The advantage of this model is that it allows us to measure 4 phenotypic traits suggested as predisposing factors related to vulnerability to addiction. Also, it is possible to evaluate the long food addiction mouse model with mice genetically modified. Importantly, the novelty of this protocol is the adaptation of this food addiction model to a short protocol to evaluate genetic manipulations targeting specific brain circuitries by using a chemogenetic approach that could promote the rapid development of this addictive behavior. These adaptations lead to a short food addiction mouse protocol, in which mice follow the same behavioral procedure of the early period in the long food addiction protocol with some variations due to the surgical viral vector injection. To our knowledge, there is no paradigm in mice allowing us to study the combination of such a robust behavioral approach that allows uncovering the neurobiology of food addiction at the brain circuit level. We can study using this protocol if modifying the excitability of a specific brain network confers resilience or vulnerability to developing food addiction. Understanding these neurobiological mechanisms is expected to help to find novel and efficient interventions to battle food addiction.
Keywords: Food addictionBackground
In the last years, food addiction has gained attention due to the increasing prevalence worldwide (19.9 %) and currently represents a high cost to the individual and the society without any effective treatment available (Pursey et al., 2014). The current diagnosis is performed by a recently validated tool, the Yale Food Addiction Scale 2.0 (YFAS 2.0). This instrument is based on the criteria applied in the 5th edition of the Statistical Manual of Mental Disorders (DSM-5) for substance use disorders, taking into account the increasing evidence suggesting that food addiction shares its neurobiological substrates with drug addiction (Lindgren et al., 2017). Food addiction is a complex multifactorial brain disorder resulting from the dynamic interaction among multiple gene networks and multiple environmental factors impacting brain development and function, leading to individual differences among the population (Hamer, 2002; Nestler et al., 2015). For this reason, not all individuals become addicted and extreme subpopulations can be distinguished with an addicted and non-addicted phenotype (Piazza and Deroche-Gamonet, 2013). Conversely, the precise neurobiological mechanisms underlying both phenotypes are still unclear despite the well-known common brain areas involved in addictive processes that include the basal ganglia, extended amygdala, and prefrontal cortex (Koob and Volkow, 2016; Moore et al., 2017). The current protocol improves previous studies because it has the inclusion of a short protocol for evaluating food addiction phenotype in genetically modified mice that present anticipation of food addiction development. In this protocol, the development of loss of control over food intake that characterizes addiction is revealed by measuring compulsivity, motivation, and persistence in different time-points. Compared to other operant models, this has the advantage of measuring other phenotypic traits such as impulsivity, cognitive flexibility, appetitive associative learning, and aversive conditioning. These traits are potential predictors of the development of food addiction. In this study, the main aim is to describe a replicable protocol that allows deciphering the neurobiological mechanisms involved in the resilient and vulnerable phenotypes to develop a food addiction. To address this major question, we describe a protocol with a reliable behavioral approach that can be adapted to combine a viral vector approach with chemogenetic manipulations. These findings will help to design new strategies to focus the strength in the prevention of the transition to food addiction by increasing the inhibitory control of individuals exposed to unhealthy environmental conditions.
Materials and Reagents
Materials
- Chocolate-flavored pellets (20 mg/pellet, 5TUL #1811223, TestDiet, Richmond, IN, USA)
- Microsyringe (10 μl, Model 1701 N S.Y.R., Cemented N.D.L., 26 ga, 2 in, point style 3, #80039, Hamilton company, N.V., U.S.A.)
- Polyethylene tubing (PE-20, #C315CT , Plastics One, U.K.)
- Bilateral guide cannula (26-gauge cannula cut 12 mm below pedestal, #C235GS-5/Spc, Plastics One, U.K.)
- Bilateral internal cannula (33-gauge internal cannula fits 12 mm C235GS-5/Spc with 3 mm projection, Plastics One, U.K.)
- Osmotic minipumps (flow rate of 0.25 μl/h for 28 days, Model 2004, #0000298 , Alzet, CA, U.S.A.)
- Scalpel ( #02-036-040 , AgnTho's, Sweden)
- Manual drill ( DH-1 , Plastics One, U.K.)
- Blunt-tipped surgical scissors ( #03-022-105 , AgnTho's, Sweden)
- Curved iris forceps ( #08-513-005 , AgnTho's, Sweden)
- Suture clips ( #08-922-125 , AgnTho's, Sweden)
- Surgical clips ( #22-620-007 , AgnTho's, Sweden)
- Suture thread (black braided silk, TB10, 3/8 TRIANG 15 mm 4/0 90 cm, #55327-50U , LorcaMarín, Spain)
Reagents
- Distilled water
- Ethanol 70%
- Iodine (Betadine, 500 ml, #716720 , MEDA Pharma S.A.U., Spain)
- Physiological saline (0.9%, 250 ml, #999790.8 , Laboratorios ERN, Spain)
- Glucose serum (GlucosaVet 5g/100ml, #1248 ESP , B. Braun Vet Care, Spain)
- Xilin night (5 g, #2919-PS-CM , Visufarma, Spain)
- Blastoestimulina (1%, 30g, # 719385 , Almirall, Spain)
- Vetflurane (Isoflurane, 250 ml, #2199-ESP , Virbac, Spain)
- Clozapine N-oxide (CNO, 25 mg, #BML-NS105-0025 , Enzo Life Sciences, NY) diluted in 0.9% sterile saline (5 mg/ml)
- Anesthesia reagents
- Ketamine hydrochloride (75 mg/kg of body weight, 10 ml, Ketamidor, #580393 , Richterpharma ag, Austria) dissolved in sterile 0.9% physiological saline
- Medetomidine hydrochloride (1 mg/kg of body weight, #570686 , Domtor; Esteve, Spain) dissolved in sterile 0.9% physiological saline
- Atipamezole hydrochloride (2.5 mg/kg of body weight, #570559 , Revertor; Virbac, Spain) dissolved in sterile 0.9% physiological saline
- Gentamicine (1 mg/kg of body weight, #999037 , Genta-Gobens; Laboratorios Normon, Spain) dissolved in sterile 0.9% physiological saline
- Meloxicam (2 mg/kg of body weight, Metacam; #059/02/08CVFPT , Boehringer Ingelheim, Rhein) dissolved in sterile 0.9% physiological saline
- Viral vectors (storage at -80 °C). Examples
- AAV8-hSyn-DIO-hM4D(Gi)-mCherry (1.21E + 13 gc/ml, Viral Vector Production Unit of Universitat Autònoma de Barcelona)
- AAV8-hSyn-DIO-mCherry (1.19E + 13 gc/ml, Viral Vector Production Unit of Universitat Autònoma de Barcelona)
- AAVrg pmSyn1-EBFP-Cre (6 x 1012 vg/ml, Addgene, viral prep # 51507-AAVrg )
Equipment
- Mouse operant self-administration chambers (Model ENV-307A-CT , Med Associates, Georgia, VT, U.S.A.)
The operant chambers are equipped with two retractable levers (#ENV-312-2M, Med Associates), one randomly selected as the active lever and the other as the inactive. Pressing on the active lever results in a food pellet delivery paired with a stimulus-light (associated-cue, #ENV-321M, Med Associates), located above the active lever, and while pressing on the inactive lever has no consequences. A food dispenser (#ENV-303M pellet receptacle, #ENV-203M-20, modular pellet dispenser, Med Associates) equidistant between the two levers permit the delivery of food pellets when required. The floor of the chambers is a grid floor (#ENV-307A-GF, Med Associates) that serves to deliver electric foot shocks in the session of shock test and serves as a contextual cue in the session of shock-induced suppression the day after the shock test. During the rest of the self-administration sessions, a metal sheet with holes is placed above the grid floor. Thus, mice can discriminate between different contexts. A house light is placed on the ceiling of the chamber (#ENV-315M, Med Associates). The chambers are made of aluminum and acrylic and are housed in sound- and light-attenuated boxes equipped with fans to provide ventilation and white noise. (Figure 1).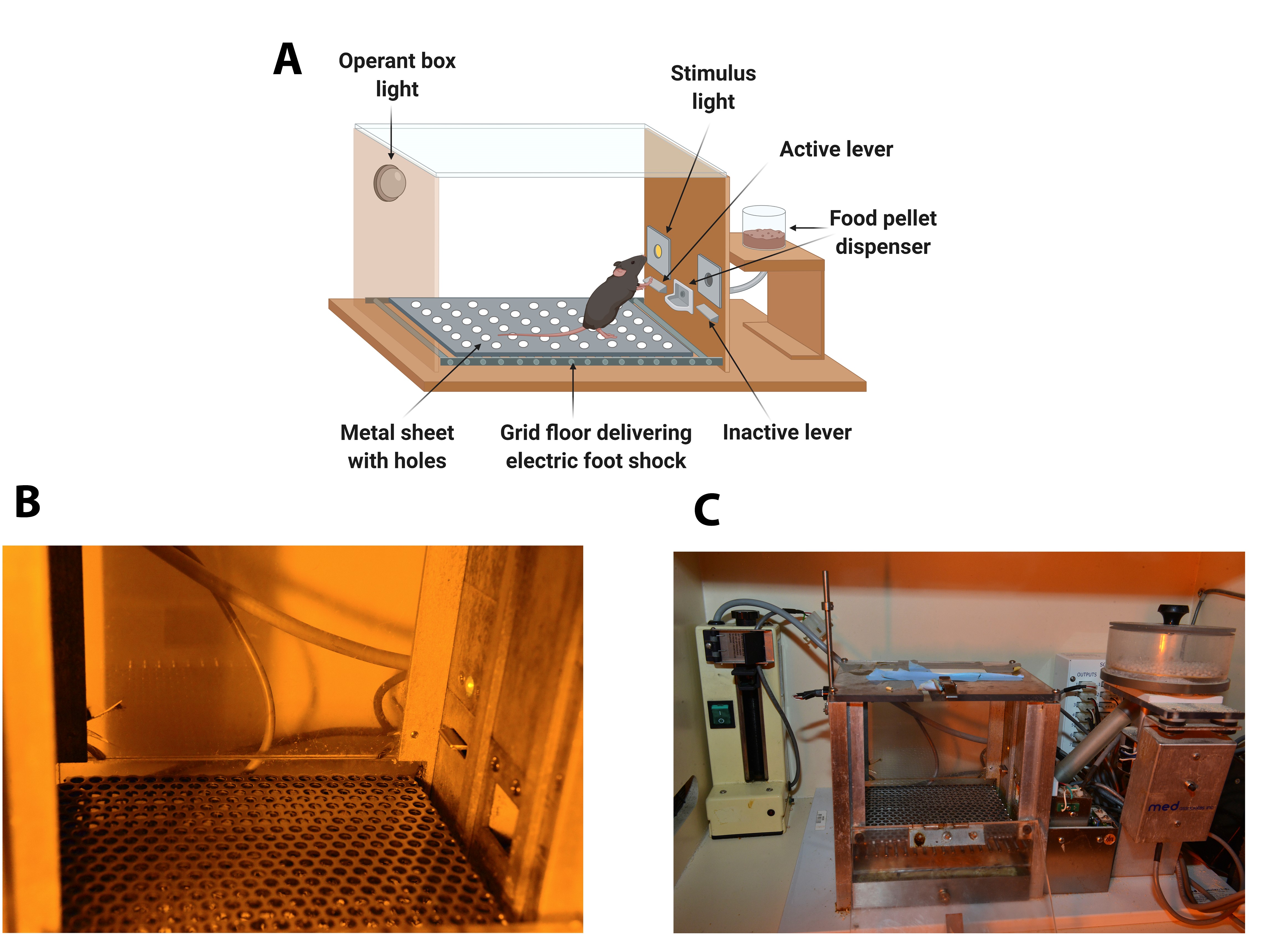
Figure 1. Diagram and images of an operant self-administration chamber. A. Diagram of the operant chamber. The operant chamber is equipped with two retractable levers (active and inactive), a stimulus-light, and a food pellet dispenser. The floor of the chamber is a grid floor that delivers electric foot shocks in the session of shock test but is covered during the rest of self-administration sessions with a metal sheet with holes. B. Image with detail of the panel containing the active lever signaled by the cue-light and the metal sheet with holes. C. General view of the Skinner Box with the operant box light, the active lever, the cue-light, the grid floor that delivers electric foot shocks, the metal sheet with holes, and the food dispenser. - Stereotaxic apparatus (100-micron resolution, Model 900 , Koft instruments, C. A., U.S.A.)
- Standing magnifier (OPMI 1 FR, Carl Zeiss, U.S. A.)
- Microinfusion pump (P.H.D. 2000, #MA1 70-20xx , Harvard Apparatus, Holliston, MA, U.S.A.)
- Animal trimmer ( #M630 , Artero, Spain)
- Cold light (Leica C.L.S. 150x, Leica Microsystems, Spain)
- Heating pad ( #N2P 220-230 , 60W, 50Hz, Daga, Spain)
- Hot bead sterilizer (FST 250, #18000-45 , AgnTho's, Sweden)
Software
- Med-PC Software (Med Associates Inc, U.S.A.). Software that registers all the behavior in the operant self-administration chambers
- GraphPad Prism software (GraphPad Software, U.S.A.) to perform all graphs
- SPSS software (I.B.M., version 25) to perform statistical data analysis
Procedure
Male mice are housed individually in temperature (21 ± 1 °C)-and humidity (55 ± 10%) -controlled laboratory conditions maintained with food and water ad libitum. Mice are tested during the dark phase of a reverse light cycle (lights off at 8.00 a.m and on at 8.00 p.m).
- Self-administration session
The beginning of each self-administration session is signaled by turning on a house light placed on the ceiling of the chamber during the first 3 s. Daily self-administration sessions maintained by chocolate-flavored pellets last 1 h in the long food addiction protocol and 2 h in the short food addiction protocol to increase the exposure of the palatable pellets on each day to ensure the development of the addiction-like phenotype. The self-administration sessions are composed of 2 pellet periods (25 min and 55 min) separated by a pellet-free period (10 min). During the pellet periods, pellets are delivered contingently after an active response paired with a stimulus light (cue light). A time-out period of 10 s is established after each pellet delivery, where the cue light is off, and no reinforcer is provided after responding on the active lever. Responses on the active lever and all the responses performed during the time-out period are recorded. During the pellet-free period, no pellet is delivered, and this period is signaled by the illumination of the entire self-administration chamber. After each session, mice are returned to their home cages.
In the operant conditioning sessions, mice are under a fixed ratio 1 (FR1) schedule of reinforcement (1 lever-press results in one pellet delivery) followed by an increased F.R. to 5 (FR5) (5 lever-presses results in 1 pellet delivery) for the rest of the sessions. As previously described (Martín-García et al., 2011), the criteria for the achievement of the operant responding are acquired when all of the following conditions are met: (1) mice maintained a stable responding with less than 20% deviation from the mean of the total number of reinforcers earned in 3 consecutive sessions (80% of stability); (2) at least 75% responding on the active lever; and (3) a minimum of 5 reinforcers per session. - Measurement of the 3 food addiction-like criteria
Three behavioral tests are used to evaluate the food addiction-like criteria as described (Mancino et al., 2015; Domingo-Rodriguez et al., 2020) and adapted from cocaine addiction-like in rats (Deroche-Gamonet et al., 2004). These 3 criteria summarize the hallmarks of addiction based on DSM-IV (Piazza and Deroche-Gamonet, 2013), specified in DSM-5, and now included in the food addiction diagnosis through the YFAS 2.0 (Gearhardt et al., 2016).- Persistence to response (Figure 2A): Non-reinforced active responses during the pellet free period (10 min), when the box is illuminated and signaling the unavailability of pellet delivery, are measured as persistence of food-seeking behavior. On the 3 consecutive days before the progressive ratio, mice are scored.
- Motivation (Figure 2B): The progressive ratio schedule of reinforcement is used to evaluate the motivation for chocolate-flavored pellets. The response required to earn one single pellet escalates according to the following series: 1, 5, 12, 21, 33, 51, 75, 90, 120, 155, 180, 225, 260, 300, 350, 410, 465, 540, 630, 730, 850, 1,000, 1,200, 1,500, 1,800, 2,100, 2,400, 2,700, 3,000, 3,400, 3,800, 4,200, 4,600, 5,000, and 5,500. The maximal number of responses that the animal performs to obtain one pellet is the last event completed, referred to as the breaking point. The maximum duration of the progressive ratio session is 5 h or until mice do not respond on any lever within 1 h.
- Compulsivity (Figure 2C): Total number of shocks in the session of shock test (50 min), when each pellet delivered is associated with punishment, are used to evaluate compulsivity-like behavior, previously described as resistance to punishment (Deroche-Gamonet et al., 2004; Mancino et al., 2015). Mice are placed in a self-administration chamber without the metal sheet with holes and, consequently, with the grid floor exposed (contextual cue). In this shock-session, mice are under an FR5 schedule of reinforcement during 50 min with 2 schedule changes: at the 4th active lever-response mice receive only an electric foot-shock (0.18 mA, 2 s) without pellet delivery, and at the 5th active lever-response, mice receive another electric foot-shock with a chocolate-flavored pellet paired with the cue light. The schedule is reinitiated after 10 s pellet delivery (time-out period) and after the 4th response if mice do not perform the 5th response within 1 min.
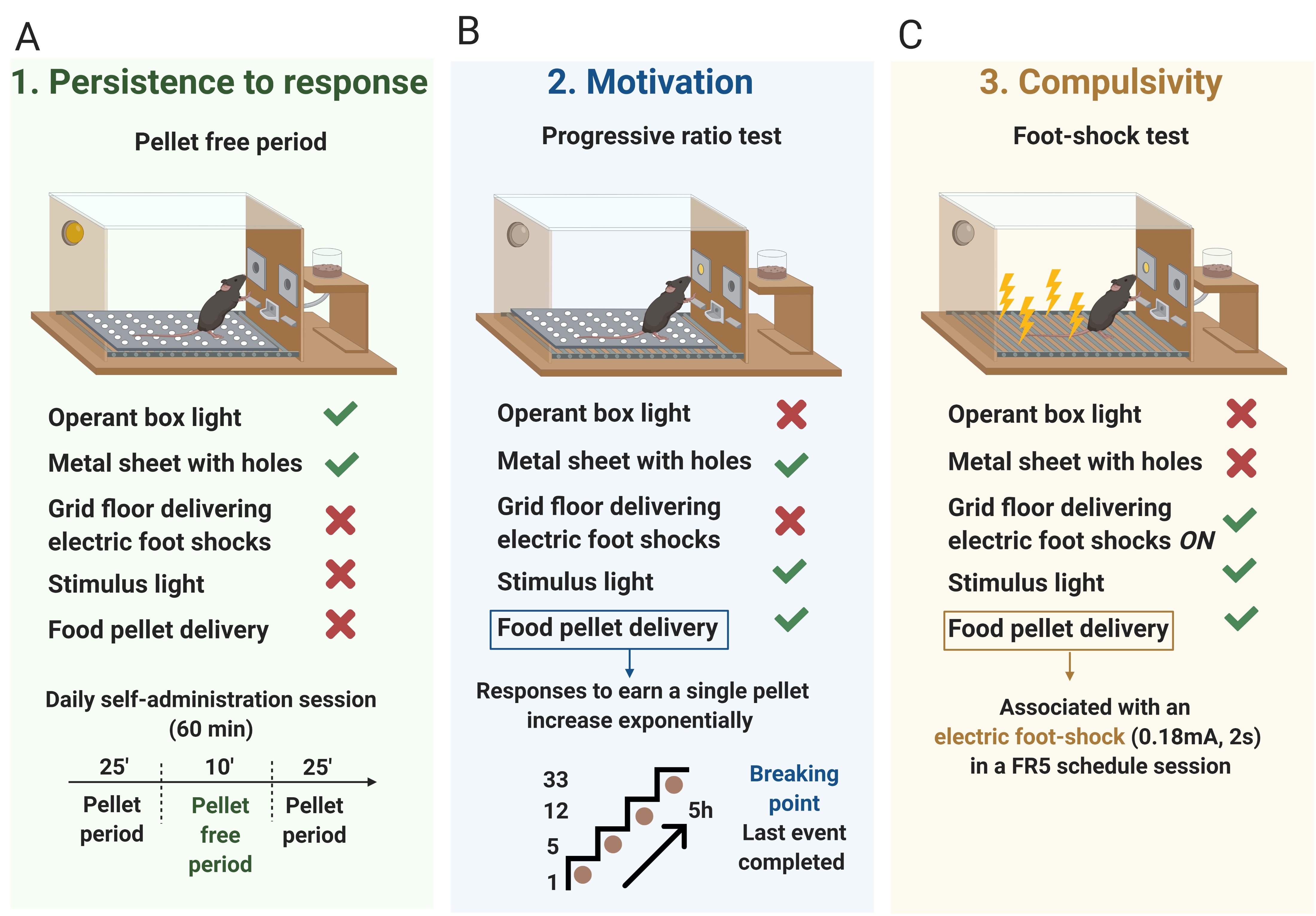
Figure 2. The 3 addiction-like criteria. A. Persistence to response. Non-reinforced active responses during the pellet free period (10 min), when the box is illuminated and signaling the unavailability of pellet delivery, are used to evaluate the persistence of food-seeking. B. Motivation. The progressive ratio test is used to evaluate the motivation for chocolate-flavored pellets. The responses to earn a single pellet increase exponentially. The maximal number of responses that the animal performs to obtain one pellet is the last event completed, referred to as the breaking point. C. Compulsivity. The total number of shocks in the session of shock test, when each pellet delivered is associated with punishment, is used to evaluate compulsivity-like behavior. - Attribution of the 3 addiction-like criteria (Figure 3): After performing the 3 behavioral tests to measure the food addiction-like behavior, mice are categorized in vulnerable or resilient animals at the early period and addicted or non-addicted animals at the late period, depending on the number of positive criteria that they have achieved at the early or late period respectively. An animal is considered positive for an addiction-like criterion when the score of the specific behavioral test is equal or above the 75th percentile of the normal distribution of the chocolate control group. Mice that achieve 2 or 3 addiction-like criteria are considered vulnerable, or addicted animals and mice that achieve 0 or 1 addiction-like criteria are considered resilient or non-addicted animals.
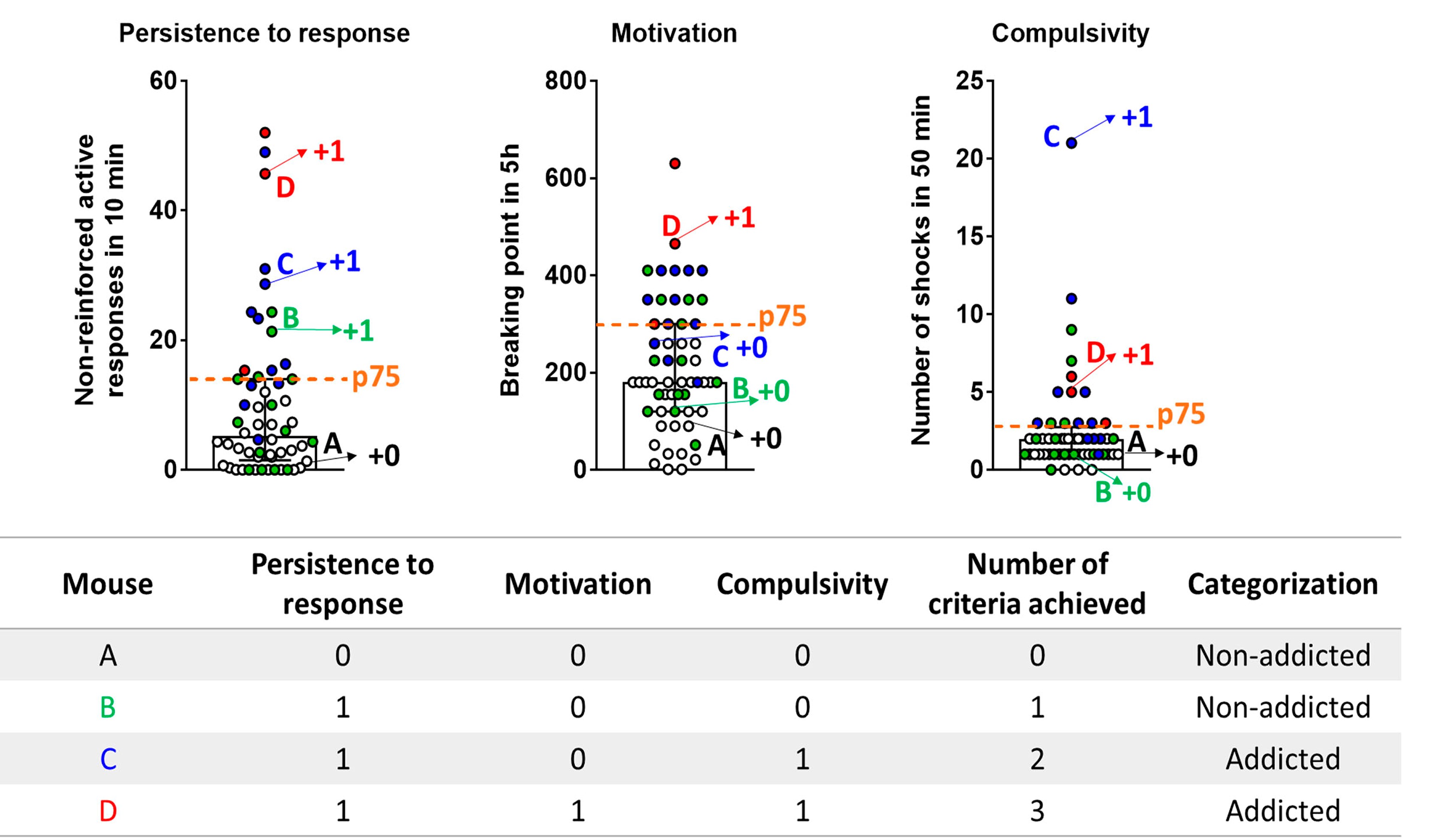
Figure 3. Attribution of the 3 addiction-like criteria Mice perform 3 behavioral tests to measure the food addiction-like behavior and obtain an individual score for each criterion. The percentile 75th of the normal distribution of the chocolate control group in each criterion (dashed horizontal line) is established as a threshold to consider an animal positive for this addiction-like criterion when its individual score is equal or above the 75th percentile. Mice that achieve 2 or 3 addiction-like criteria are considered addicted animals, and mice that achieve 0 or 1 addiction-like criteria are considered non-addicted animals. Four mice (A-D) are indicated in the figure as an example. Mouse A presents the values of each criterion below the threshold achieving 0 criteria and is classified as a non-addicted animal. Mouse B displays a score in persistence to the response above the threshold and below in motivation and compulsivity, achieving 1 criterion and is classified as a non-addicted animal. Mouse C shows a score in persistence to response and compulsivity above the threshold and below in motivation achieving 2 criteria and is categorized as an addicted animal. Mouse D shows a score of each criterion above the 75th percentile achieving 3 criteria and is classified as an addicted animal. Data are expressed as individual values and median with interquartile range. White circles: mice with 0 criteria. Green circles: mice with 1 criterion. Blue circles: Mice with 2 criteria. Red circles: mice with 3 criteria. Data derived from Domingo-Rodriguez et al., 2020. - Measurement of 4 phenotypic traits considered as factors of vulnerability to addiction-like behavior
- Impulsivity (Figure 4A): Non-reinforced active responses during the time-out periods (10 s) after each pellet delivery are measured as impulsivity-like behavior indicating the inability to stop a response once it is initiated (Logan et al., 1997; Koob and Volkow, 2010). The 3 consecutive days before the progressive ratio test are considered for this criterion.
- Cognitive flexibility (Figure 4B): Measured with a reversal test that indicates the ability to change responding to stimuli that have previously predicted the availability of reward (Schoenbaum et al., 2011). The reversal test is a standard training self-administration session, but the active and the inactive levers are reversed.
- Appetitive associative learning (Figure 4C): Measured with the cue-induced food seeking test. The cue-induced food-seeking test lasts 90 min and is divided into two periods: 60 min + 30 min. In the first 60 min period, all lever-presses are not reinforced (active and inactive lever-presses have no scheduled consequences). In the subsequent 30 min, the white cue light previously associated with pellet delivery during a normal self-administration session is illuminated contingently for 30 min according to an FR5. To signal the change in the schedule, the cue light is presented twice non-contingently and for 4 s.
- Aversive associative learning (Figure 4D): Measured with the shock-induced suppression test. In the shock-induced suppression test, mice are placed in the self-administration chamber for 50 min with the same grid floor used during the shock-test. However, during this FR5 self-administration session, pressing the active lever has no consequences, no shock, no chocolate-flavored pellets, and no cue-light. Non-reinforced active responses during this shock-induced suppression test are measured for the aversive associative learning.
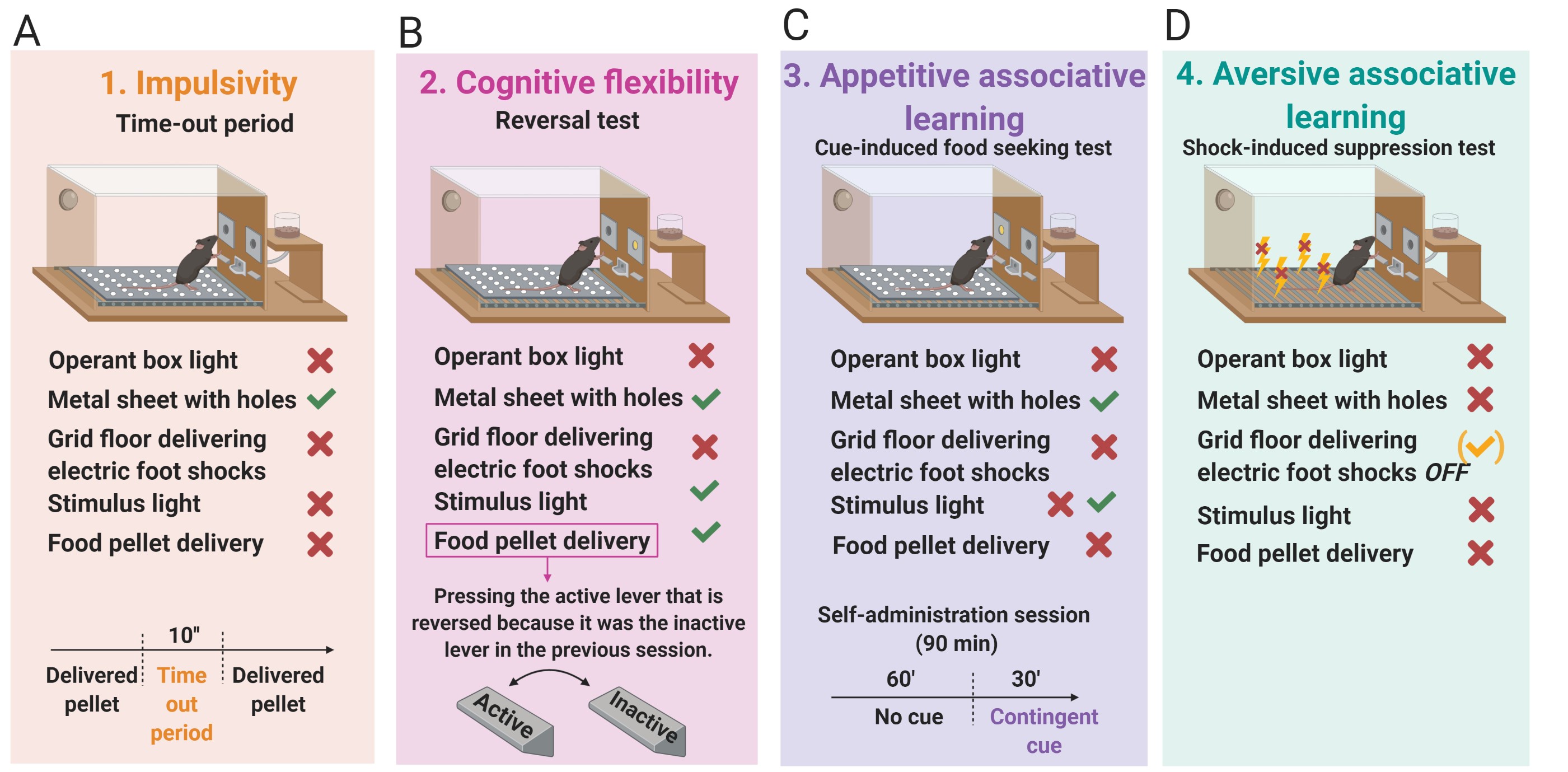
Figure 4. The 4 phenotypic traits considered as factors of vulnerability to addiction-like behavior. A. Impulsivity measured by the non-reinforced active responses not paired with a stimulus light during the time-out periods (10 s) after each pellet delivery. B. Cognitive flexibility measured by the reversal test. The reversal test is an FR5 self-administration session, but the active and the inactive levers are reversed compared to the previous self-administration session. C. Appetitive associative learning measured by the cue-induced food-seeking test. The cue-induced food-seeking test is a self-administration session that longs 90 min and is divided into two periods: 60 min + 30 min. In the first 60 min period, all lever-presses are not reinforced (active and inactive lever-presses have no scheduled consequences). In the subsequent 30 min, the white cue light, associated with pellet delivery during a normal self-administration session, is illuminated contingently for 30 min according to an FR5. D. Aversive associative learning measured by the shock-induced suppression test. - Experimental design
Long protocol for food addiction in mice (Figure 5): In the long food protocol, mice are trained under FR1 schedule of reinforcement during 6 sessions, followed by 112 sessions of FR5 to self-administer chocolate-flavored pellets. During FR5 sessions, the 3 addiction-like criteria (1) persistence to response (2), motivation (3), and compulsivity and 4 phenotypic traits considered as factors of vulnerability to addiction (1) impulsivity, (2) cognitive flexibility, (3) appetitive associative learning and (4) aversive associative learning, are evaluated at 2 different time points in each mouse. The 1st time point is the early period (sessions 1-18 of FR5), and the 2nd time point is the late period (sessions 95-112 of FR5). Depending on the positive criteria that mice have achieved in the early period, animals are categorized in resilient (0-1 criteria) or vulnerable animals (2-3 criteria), and in the late period, mice are categorized in non-addicted (0-1 criteria) or addicted animals (2-3 criteria).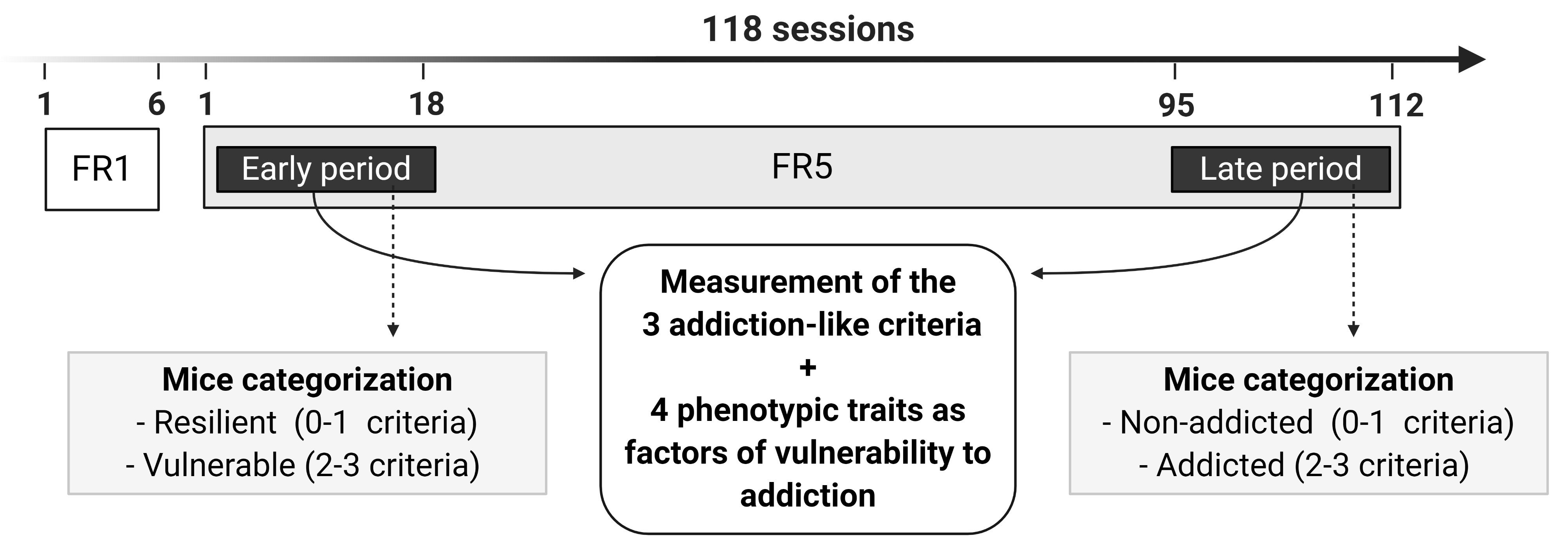
Figure 5. Timeline of the experimental sequence of the long food addiction mouse model. Mice are trained for chocolate-flavored pellets under an FR1 schedule of reinforcement on 1 h daily sessions for 6 days, followed by 112 days on an FR5. In the FR5, 2-time points are considered, early and late period, to measure the 3 addiction-like criteria (persistence to response, motivation, and compulsivity). Depending on the positive criteria that mice have achieved in the early period, animals are categorized in resilient (0-1 criteria) or vulnerable animals (2-3 criteria), and in the late period, mice are categorized in non-addicted (0-1 criteria) or addicted animals (2-3 criteria). In both early and late periods, 4 phenotypic traits as factors of vulnerability to addiction (impulsivity, cognitive flexibility, appetitive associative learning, and aversive associative learning) are also evaluated. - Experimental design
Short protocol for food addiction in mice (Figure 6): In the short protocol, mice follow the same behavioral procedure described for the early period in the long food addiction protocol with some variations due to the surgical viral vector injection. The short protocol lasts 9 weeks. In the 1st week, mice are trained to acquire the operant conditioning maintained by chocolate-flavored pellets under FR1 (2 sessions) and FR5 (2 sessions) schedules of reinforcement. In the 2nd week, the viral vector of interest is injected in mice by stereotaxic surgery.
Examples of viral vectors strategies:- Chemogenetic approach (DREADD approach): It refers to the injection of Cre-dependent AAV carrying a DREADD in a determined brain region of genetically modified mice expressing the Cre-recombinase in a specific cell-type.
- Combined chemogenetic and a retrograde AAV approach (retro-DREADD approach): It refers to the combinatorial injection of Cre-dependent AAV carrying a DREADD in a determined brain region and an AAV retrograde expressing the Cre-recombinase in its projecting brain area to target a specific network in wild type mice.
After bilateral intracranial injection/s of the viral vector/s, the expression of the viral vector/s is allowed during the period of 4 weeks (2nd, 3rd, 4th, and the 5th week). At the beginning of this period (3rd week), mice are under FR5 (4 sessions) to recover the basal levels of operant responding. At the end of these 4 weeks (5th week), an osmotic minipump filled with CNO or saline is subcutaneously implanted in the back of each mouse. Subsequently, during the 6th, 7th, 8th, and 9th weeks, when it is a chronically CNO-induced activation of the expressed DREADD receptors, mice are under FR5 scheduled sessions followed by the measurement of the 3 addiction-like criteria. Finally, depending on the positive criteria that mice have achieved, animals are categorized in non-addicted (0-1 criteria) or addicted animals (2-3 criteria).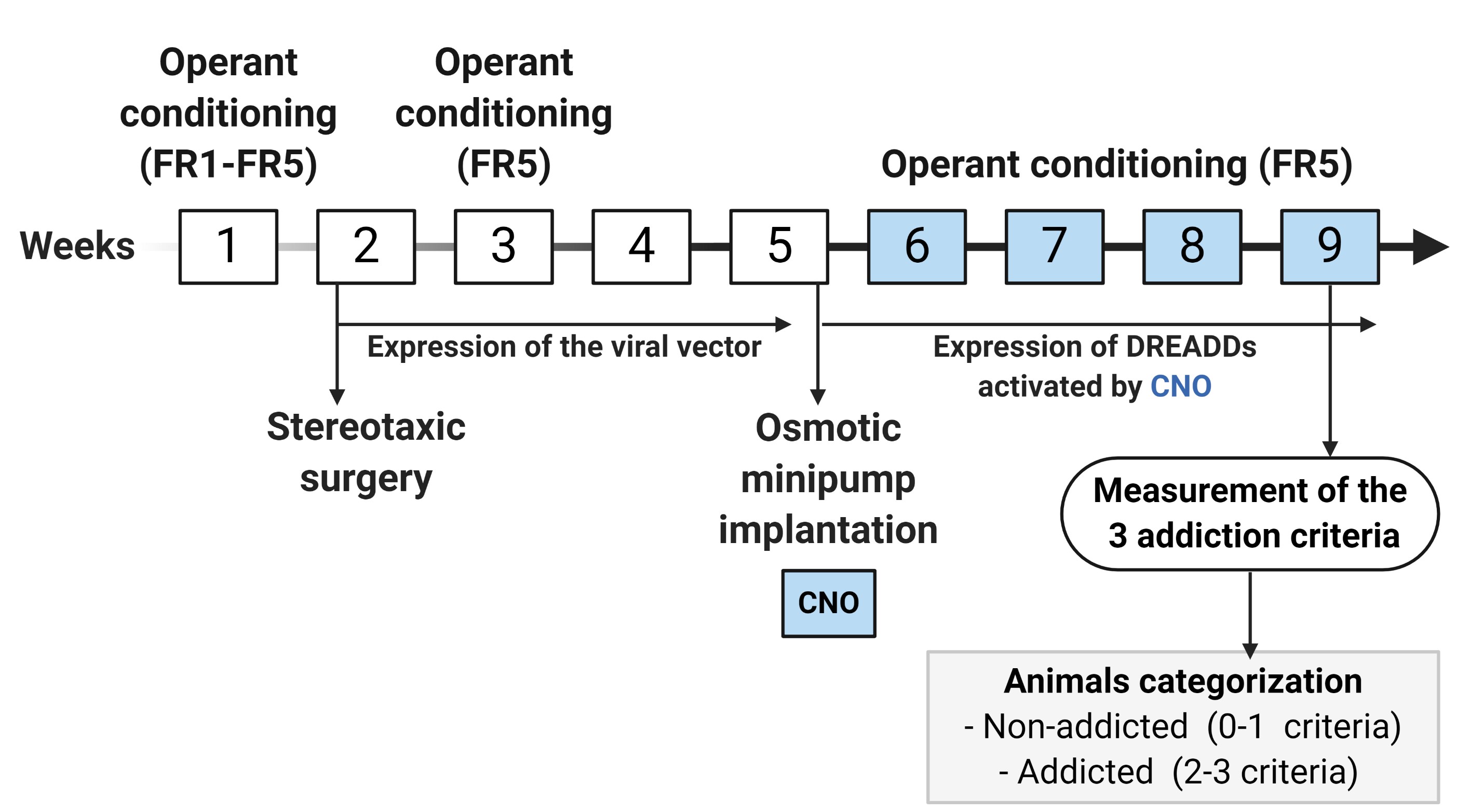
Figure 6. Timeline of the experimental sequence of the short food addiction mouse model. In the 1st week, mice are trained to acquire the operant conditioning maintained by chocolate-flavored pellets under FR1 (2 sessions) and FR5 (3 sessions) schedule of reinforcement followed by the surgery for injecting the viral vector of interest (2nd week). After surgery, the expression of the viral vector is allowed for a period of 4 weeks (2nd, 3rd, 4th, and the 5thweek). At the beginning of this period (3rd week), mice are nder FR5 (4 sessions) to recover the basal levels of operant responding, and at the end of this period (5th week), an osmotic minipump filled with CNO is implanted. During the 6th, 7th, 8th, and 9th weeks, when it is a chronic inhibition of the CNO-induced activation of the expressed DREADD receptors, mice are under FR5 scheduled sessions followed by the measurement of the 3 addiction-like criteria.
- Long food addiction mouse protocol
- Sessions 1-6 (FR1): During these sessions, operant conditioning under the FR1 schedule of reinforcement was applied.
- Sessions 1-112 (FR5): During these sessions, operant conditioning under the FR5 schedule of reinforcement was applied.
- Sessions 1-18 (FR5): These sessions of FR5 constituted the early period of the long food addiction mouse protocol.
- Sessions 3-5: FR5 sessions to evaluate the criterion of persistence to response and the phenotypic trait of impulsivity were applied.
- Session 6: Progressive ratio test to evaluate the motivation criteria.
- Session 10: Shock test to evaluate the compulsivity criteria.
- Session 11: Shock-induced suppression test to evaluate the aversive associative learning phenotypic trait.
- Session 15: Cue-induced food-seeking test to evaluate appetitive associative learning phenotypic trait.
- Session 18: Reversal test to evaluate cognitive flexibility phenotypic trait.
- Categorize mice in vulnerable and resilient animals depending on the number of positive addiction-like criteria achieved at the early period.
- Sessions 18-95 (FR5): FR5 sessions.
- Sessions 95-112 (FR5): Theses sessions constitute the late period.
- Sessions 97-99: Normal FR5 sessions to evaluate the criterion of persistence to response and the phenotypic trait of impulsivity.
- Session 100: Progressive ratio test to evaluate the motivation criteria.
- Session 104: Shock test to evaluate the compulsivity criteria.
- Session 105: Shock-induced suppression test to evaluate the aversive associative learning phenotypic trait.
- Session 109: Cue-induced food-seeking test to evaluate appetitive associative learning phenotypic trait.
- Session 112: Reversal test to evaluate cognitive flexibility phenotypic trait.
- Categorize mice in addicted and non-addicted animals depending on the number of positive addiction-like criteria achieved in the late period. Using this protocol in C57BL/6N, we obtained 25% of addicted mice.
1)The non-reinforced active responses during the pellet free period (10 min) are used to evaluate the persistence to response criteria.2)The non-reinforced active responses during the time-out periods (10 s) after each pellet delivery are used to evaluate the impulsivity phenotypic trait.1)The non-reinforced active responses during the pellet free period (10 min) are used to evaluate the persistence to response criteria.2)The non-reinforced active responses during the time-out periods (10 s) after each pellet delivery are used to evaluate the impulsivity phenotypic trait.
- Short food addiction mouse protocol
- Week 1: Learning of the operant responding to obtain chocolate-flavored pellets.
- Session 1-2: Operant conditioning under the FR1 schedule of reinforcement.
- Session 3-4: Operant conditioning under the FR5 schedule of reinforcement.
- Week 2: Injection of a viral vector by stereotaxic surgery. Stereotaxic surgery protocol (Figure 7)
- Injection cannulas circuit preparation.
- Remove the plunger of the microsyringe (10 µl).
- Attach the polyethylene tubing (50 cm) to the microsyringe.
- Inject distilled water through the tubing, using a syringe, filling the tubing, and the microsyringe. Check that the water comes out through the back of the microsyringe.
- Insert half of the plunger into the microsyringe.
- Connect one of the sides of the bilateral internal cannula (33 gauge) to the tubing. Check that the circuit is well done by pressing the microsyringe plunger and observing how the water flows out of the cannula without leakage.
- Repeat these steps with a second microsyringe, which must be attached to the other side of the same internal cannula as the first microsyringe.
- Place the microsyringes in the microinfusion pump.
- Aspirate 1 µl of air to form a bubble inside the tubing to monitor the microinjections.
- Aspirate 4 µl of the viral vector (previously defrosted with ice).
- Stereotaxic apparatus preparation.
- Place and fix the bilateral guide cannula in the metal grip of the stereotaxic holder.
- Insert the bilateral internal cannula attached to the tubing into the bilateral guide cannula. The bilateral guide cannula is used to keep the internal cannula straight and well fitted into the holder and to find the Bregma coordinates before the AAV is loaded. Note that only the internal cannula filled with the viral vector is the one that penetrates the brain.
- Mouse preparation.
- Anesthetize the mouse with ketamine hydrochloride and medetomidine hydrochloride mixed and dissolved in sterile 0.9% physiological saline. Administer intraperitoneally (75 mg/kg and 1 mg/kg of body weight, respectively).
- Shave the mouse's head.
- Place mouse in the stereotaxic apparatus. Check that the head is fixed using the ears bars and the nose clamp.
- Place a heating pad below the mouse to maintain proper body temperature during the surgery.
- Stereotaxic surgery.
- Apply iodine to the shaved head area.
- Apply xilin night on eyes to avoid keratitis.
Note: The following steps must be done using a standing magnifier.
- Perform a vertical cut in the middle of the head with a scalpel.
- Scratch the skull carefully to visualize bregma accurately.
- Place the cannulas above the bregma and annotate bregma coordinates.
- Calculate medial-lateral, dorsal-ventral, and anteroposterior coordinates of your target area where the injection has to be performed.
- Move the cannulas to the specific injection point by adjusting the stereotaxic apparatus using the medial-lateral, dorsal-ventral, and anterior-posterior readings.
- Mark the injection point on the skull using a pencil.
- Following the pencil mark, perform the holes into the skull using a manual drill.
- Clean the injection area with physiological saline.
- Insert the bilateral cannula into the holes and readjust the dorsal-ventral coordinate.
- Place precisely the bilateral cannulas following the calculated coordinates.
- Mark the two sides of the air bubble on the tubing to monitor the injection.
- Perform the viral vector injection.
- Clean the injection area with physiological saline.
- Sew the incision with suture thread.
- Apply blastoestimulina on the sutured incision to promote true healing.
- Inject subcutaneously atipamezole hydrochloride (2.5 mg/kg of body weight) dissolved in sterile 0.9% physiological saline to reverse the anesthesia.
- Inject intraperitoneally gentamicin (1 mg/kg of body weight) dissolved in sterile 0.9% physiological saline.
- Inject meloxicam subcutaneously (2 mg/kg of body weight) dissolved in sterile 0.9% physiological saline.
- Inject glucose serum (0.8 ml) subcutaneously.
- Place the animal on a heating pad until the animal is awake.
- During the following 3 postsurgery days, check the mouse and inject 0.8 ml of glucose serum every day.
- Cleaning.
- Clean the tubing and the cannulas with ethanol, followed by distilled water and air.
- Clean all the surgical material with soap and water.
After the bilateral intracranial injection, 4 weeks (weeks 2, 3, 4, and 5 in this protocol) are needed for the proper expression of the viral vector. - Week 3: Operant conditioning after surgery to recover the basal levels of responding.
Sessions 5-9: Operant conditioning under the FR5 schedule of reinforcement. - Week 4: Operant conditioning.
- Week 5: At the end of the week implant, an osmotic minipump filled with CNO or saline. Osmotic minipump implantation protocol (Figure 8)
- Anesthetize the mouse with isoflurane and fix its front and hind legs with tape.
- Shave mouse by the lower back.
- Clean the shaved lower back with ethanol.
- Make a horizontal cut (1.5 cm), taking as a reference the beginning of the hind legs.
- Separate the skin from the back muscle introducing blunt-tipped scissors through the cut.
- Introduce the osmotic minipump (ALZET 2004) previously filled with CNO (diluted in 0.9% sterile saline: 5 mg/ml) or saline subcutaneously in the middle of the back. The pump cap in the direction of the animal's head.
- Close the cut with surgical clips (around 4).
- Apply iodine to the area.
- Week 6: Operant conditioning during the expression of the viral vector activated by CNO.
Sessions 10-14: Operant conditioning under the FR5 schedule of reinforcement. - Week 7: Operant conditioning during the expression of the viral vector chronically activated by CNO.
Sessions 15-20: Operant conditioning under the FR5 schedule of reinforcement. - Week 8: Operant conditioning during the expression of the viral vector chronically activated by CNO.
- Sessions 21: Operant conditioning under the FR5 schedule of reinforcement.
- Session 22-24: Normal FR5 sessions to evaluate persistence to response and impulsivity.
- The non-reinforced active responses during the pellet free period (10 min) are used to evaluate the persistence to response criteria.
- The non-reinforced active responses during the time-out periods (10 s) after each pellet delivery are measured as impulsivity-like behavior.
- Session 25: Progressive ratio test to evaluate the motivation criteria.
- Week 9: Operant conditioning during the expression of the viral vector chronically activated by CNO.
- Sessions 26-28: Operant conditioning under FR5 schedule of reinforcement.
- Session 29: Shock test to evaluate the compulsivity criteria.
- Session 30: Shock-induced suppression test to evaluate the aversive associative learning.
- Categorize mice in addicted and non-addicted animals depending on the number of positive addiction-like criteria achieved. Using this protocol in C57BL/6J mice with an inhibition of the PL-NAc core pathway by chemogenetic approach, we obtained 50% of addicted mice.
- Verify the viral expression.
- Perfuse animals using intracardiac perfusion with 4% paraformaldehyde (P.F.A.) in 0.1 M Na2HPO4/0.1 M NaH2PO4 buffer (P.B.), pH 7.5, delivered with a peristaltic pump at 30 ml per min for 2 min. Animals are previously profoundly anesthetized by intraperitoneal injection (0.2 ml/10 g of body weight) of a mixture of ketamine/medetomidine.
- Extract the brains and post-fixed with 4% P.F.A. for 24 h and transfer them to a solution of 30% sucrose at 4 °C.
- Make coronal sections (30 μm) using a freezing microtome and store them in a 5% sucrose solution at 4 °C until immunofluorescence study.
- Perform an immunofluorescence study using the specific antibodies for your viral vector fluorescent reporter used.
- Visualize the stained sections of the brain with a confocal microscope to evaluate histological verification of correct injection placement.
- Make representative immunofluorescence pictures (Figure 9).
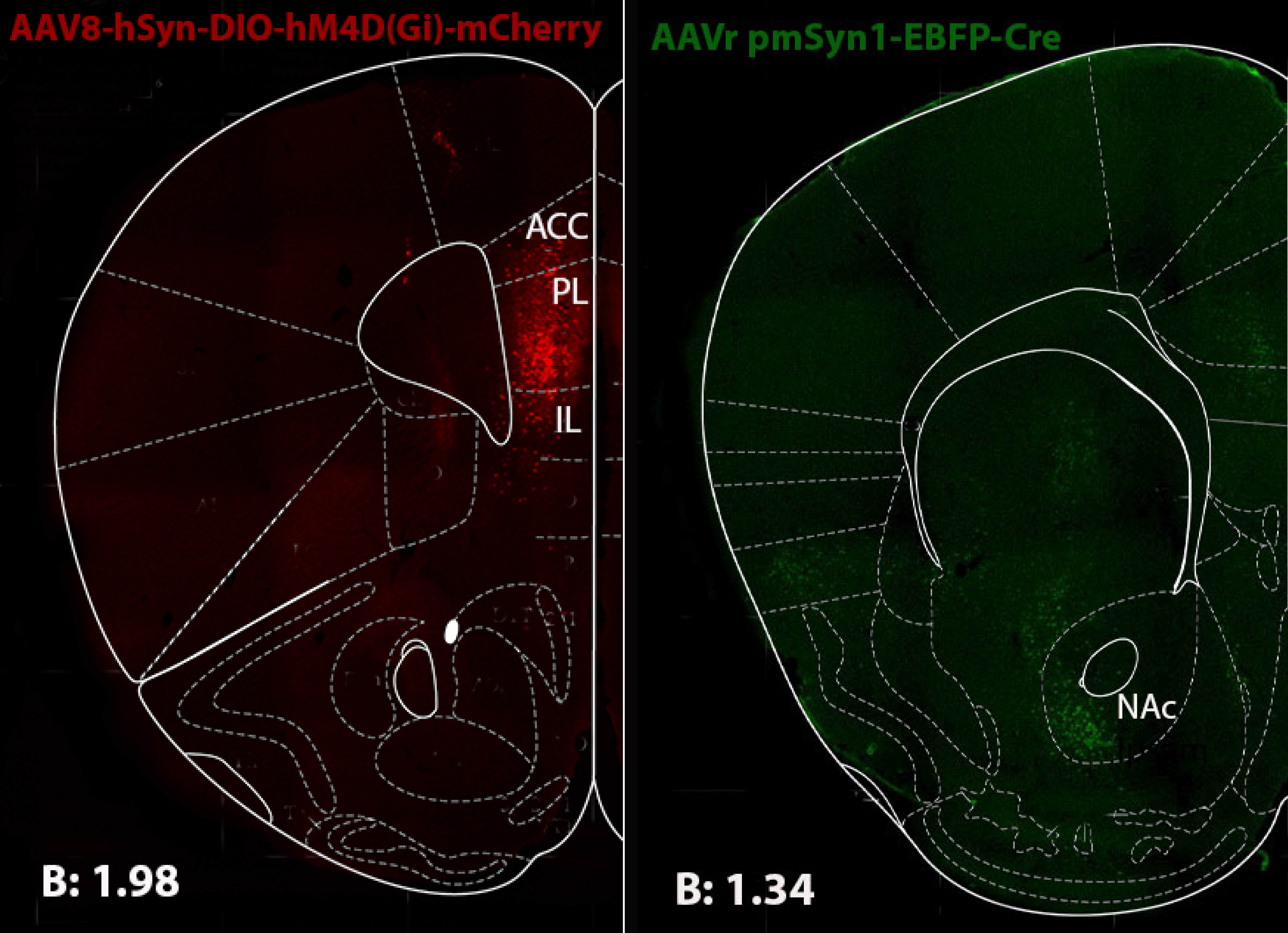
Figure 9. Representative immunofluorescence picture showing Cre-dependent hM4Di-mCherry detected at P.L. injection site (left) and Cre recombinase at NAc core (right). Data derived from Domingo-Rodriguez et al., 2020.
Note: Sterilize the surgical material with a hot bead sterilizer before each animal surgery.1)Microinfusion pump parameters (example for prelimbic (P.L.) and nucleus accumbens core (NAc core) areas)• Target volume: PL 0.2 µl; NAc core 0.4 µl
•Infusion rate: PL 0.05 µl/min; NAc core 0.1 µl/min
•Infusion time: 4 min2)Leave the cannula in place after infusion for 10 min to prevent reflux.3)Withdrawn the cannula slowly for 10 min.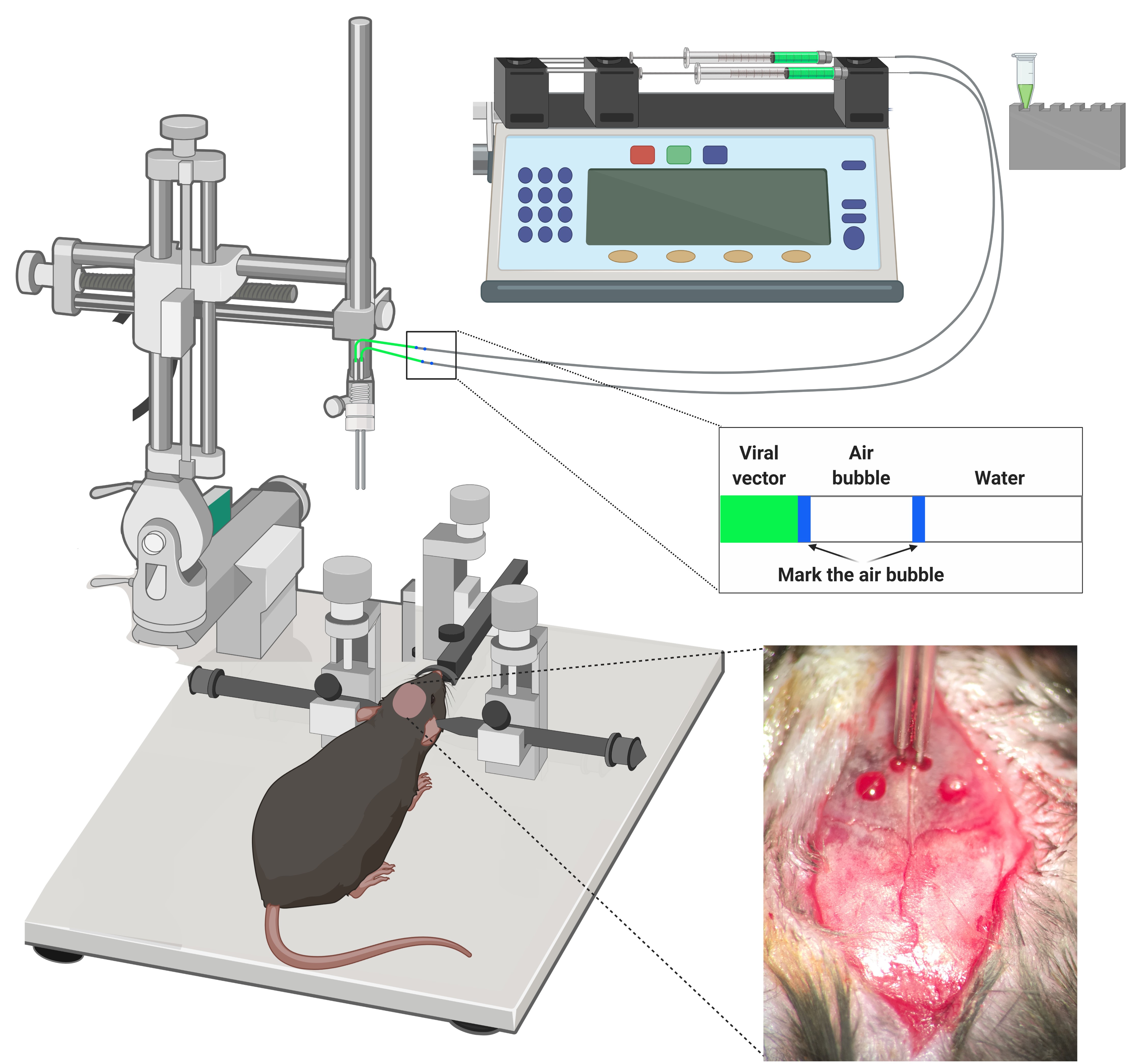
Figure 7. Stereotaxic surgery. Mice are anesthetized and placed into a stereotaxic apparatus for receiving the viral vector intracranial injections. All the injections are made through a bilateral injection cannula connected to a polyethylene. The displacement air bubble inside the length of the polyethylene tubing that connected the syringe to the injection needle is used to monitor the microinjections. The viral vector is injected at a constant rate by using a microinfusion pump. After infusion, the injection cannula is left in place for an additional period of 10 min to allow the fluid to diffuse and to prevent reflux, and then it was slowly withdrawn during 10 additional min.
Note: Frequently check the correct displacement of the air bubble inside the polyethylene tube to prevent errors of injection due to obturation of the cannula or fugues in the tube.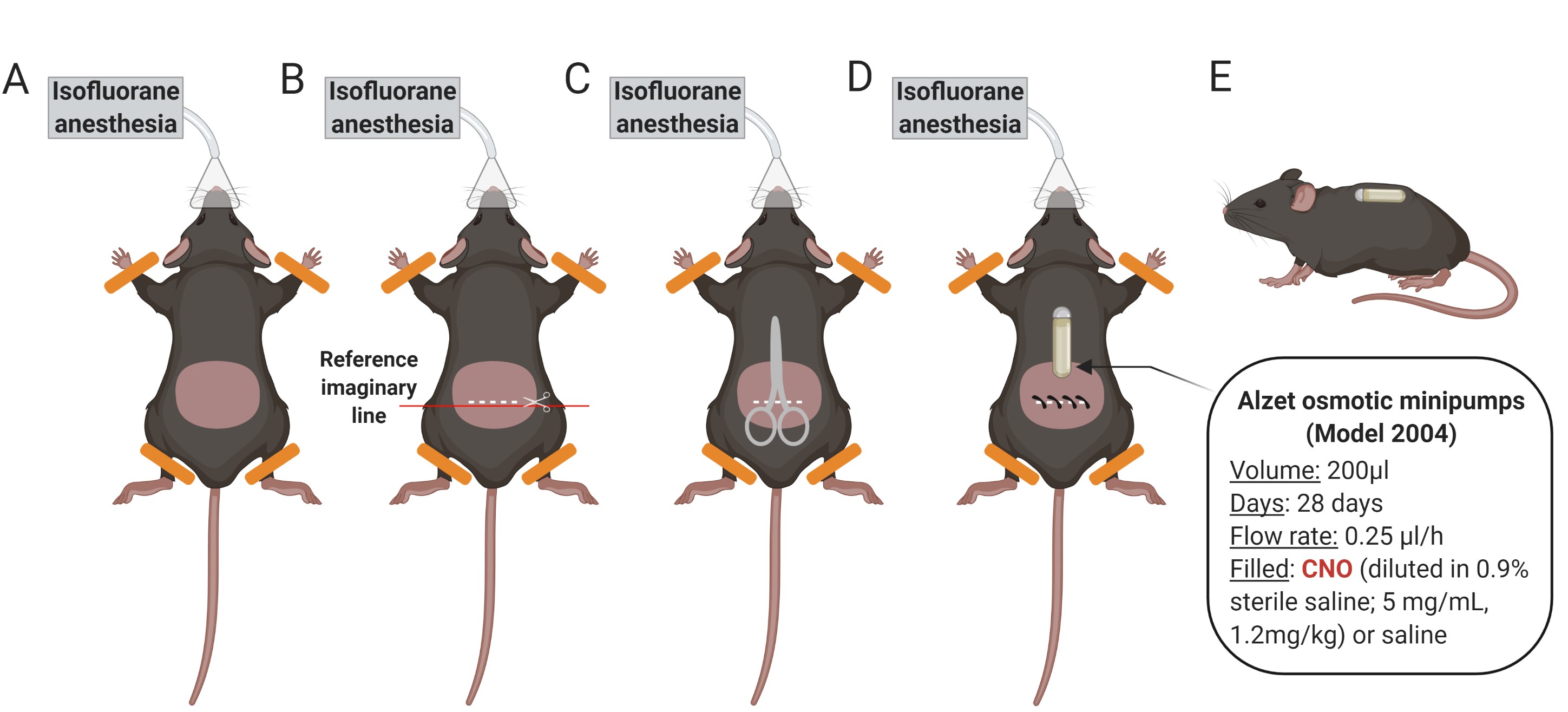
Figure 8. Schematic representation of the osmotic minipump implantation process. A. Anesthetize the mouse with isoflurane and shave it by the lower back. Clean the shaved area with ethanol. B. Make a horizontal cut (1.5 cm), taking as a reference the beginning of the hind legs. C. Separate the skin from the back muscle introducing blunt-tipped scissors through the cut. D. Introduce the osmotic minipump previously filled with CNO or saline subcutaneously in the middle of the back. The pump cap in the direction of the animal's head. Close the cut with surgical clips and apply iodine to the area. E. Side view showing the animal awake with the subcutaneously implanted minipump.
Note: Check the correct placement of the minipump the following days after surgery. If necessary, do a smooth massage in the placement site of the minipump to relocate and prevent skin wounds.Data analysis
- Exclusion criteria
- Animals that responded < 25% of all FR5 sessions and did not achieve the acquisition criteria were excluded from the remaining experimental sequence.
- Animals with a viral vector expression outside the brain regions of interest are excluded from the experiment.
- Graphs and statistical analysis
- Number of reinforcers
- Graphs: Mean number of reinforcers obtained in each session during the entire food addiction protocol per group (Figure 10). Mean ± S.E.M represents data.
- Statistical analysis: ANOVA analyzes the number of reinforcers with repeated measures for each step of the protocol separately (FR1, FR5) to test the evolution over time.
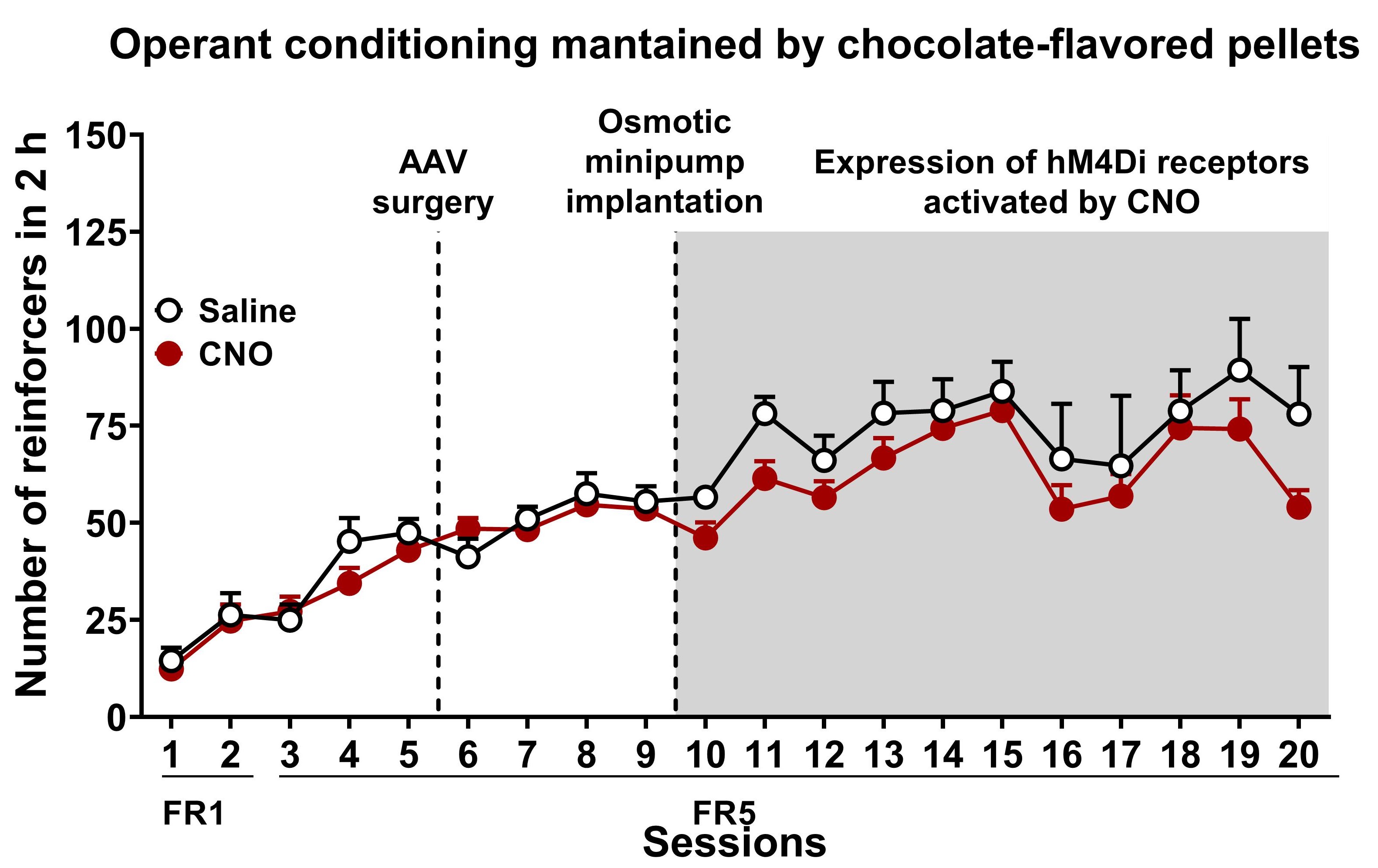
Figure 10. Chemogenetic inhibition of PL-NAc core projection leads to compulsive behavior towards highly palatable food. Number of reinforcers during operant training sessions maintained by chocolate-flavored pellets (mean ± S.E.M). In white saline-treated mice and red for CNO-treated mice (n = 12 for saline-treated mice and n = 22 for CNO-treated mice). - Behavioral tests of the 3 addiction-like criteria (Persistence to response, motivation, and compulsivity)
- Graphs: The data of the 3 criteria are expressed as individual values with the median and the interquartile range (Figure 11).
- Persistence to response: Mean of the total number of non-reinforced active responses during 3 consecutive daily 10-min of pellet free period.
- Motivation: Breaking point achieved in 5 h of the progressive ratio test.
- Compulsivity: Number of shocks that mice received in 50 min in the shock test in which each pellet delivery is associated with a foot-shock.
- The graphs of the 3 addiction-like criteria at the late period present a dashed horizontal line indicating the 75th percentile of the distribution of the control group used as the threshold to consider a mouse positive for 1 criterion.
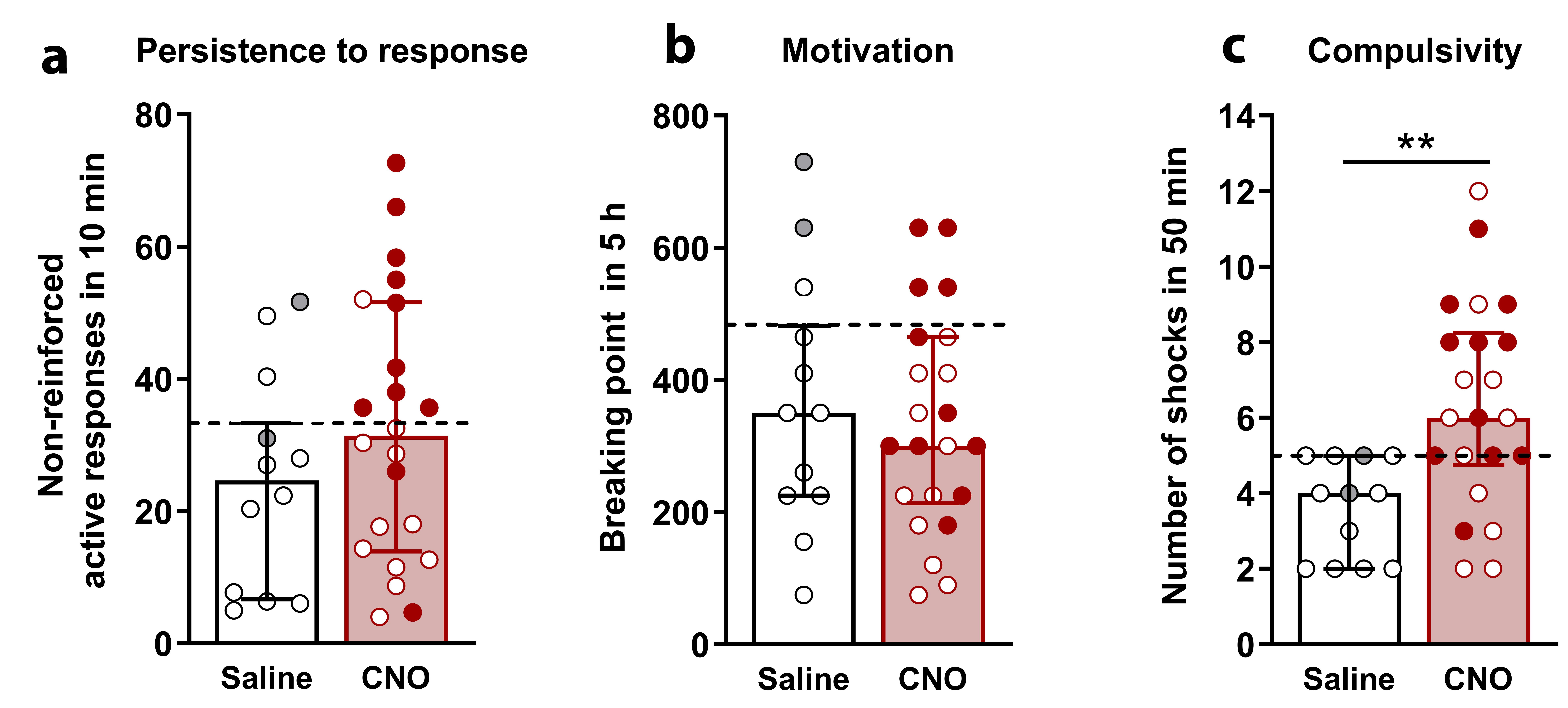
Figure 11. Behavioral tests of the three addiction-like criteria showing increased compulsivity in CNO-treated mice with an inhibition of the PL-NAc core pathway (individual values with the median and the interquartile range, U Mann-Whitney, **P < 0.01). The dashed horizontal line indicates the 75th percentile of the distribution of mice treated with saline. Addicted mice in gray filled circles for saline-treated mice and red for CNO-treated mice (n=12 for saline-treated mice and n = 22 for CNO-treated mice). - Statistical analysis
- Analyze the distribution of the sample by the Kolmogorov-Smirnov normality test.
- Comparisons between the two groups are analyzed by U Mann-Whitney if the sample does not follow a normal distribution (significance value in the Kolmogorov-Smirnov normality test).
- Comparisons between groups are analyzed by Student’s t-test if the sample follows a normal distribution (no significant value in the Kolmogorov-Smirnov normality test).
- Categorization of mice in addicted and non-addicted animals
- Graphs: Parts of holes graphs represent the percentage of addicted and non-addicted animals in each group (Figure 12).
- Statistical analysis: The percentage of addicted mice compare with the non-addicted ones are analyzed by a Chi-square test, in which it is compared the observed frequencies with the frequencies obtained in the control group.
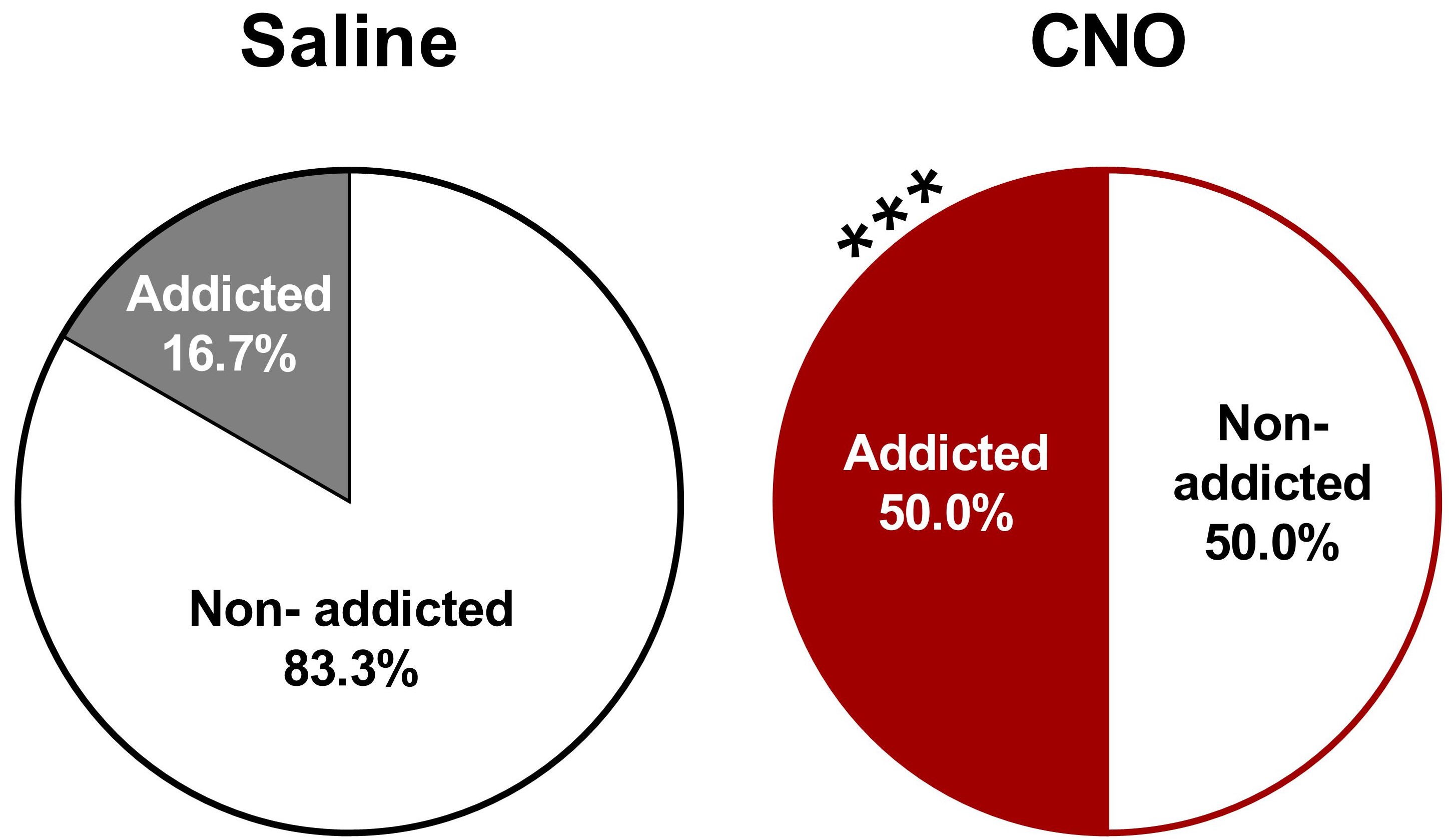
Figure 12. Increased percentage of CNO-treated with an inhibition of the PL-NAc core pathway mice classified as food addicted animals (chi-square, ***P < 0.001, n = 12 for saline-treated mice and n = 22 for CNO-treated mice). - Correlations between individual values of each addiction-like criteria and the final number of positive criteria achieved. Correlations are analyzed by Pearson's correlation coefficient.
- Behavioral tests of the 4 phenotypic traits as factors of vulnerability to addiction-like behavior (Impulsivity, cognitive flexibility, appetitive associative learning, and aversive associative learning):
- Graphs: The data of the 4 phenotypic traits are expressed as individual values with the median and the interquartile range.
- Impulsivity: Mean number of non-reinforced active responses in 50 min during 3 consecutive daily time-out (10 s) after each pellet delivery.
- Cognitive flexibility: Number of active and inactive responses in 60 min of the reversal test where active and inactive levers were reversed compared with preceding basal session.
- Appetitive associative learning:1)Active responses during 60 min period of the cue-induced food-seeking test, during which lever-presses were not reinforced, followed by active responses 30 min period during which active lever-presses (FR5) were associated with the cue-light without pellet delivery (mean ± S.E.M).2)Increase of active responses after the presentation of the cue-light.
- Aversive associative learning: Number of non-reinforced active responses in 50 min of the shock-induced suppression test.
- Statistical analysis
i.Analyze the distribution of the sample by the Kolmogorov-Smirnov normality test.
ii.Comparisons between the two groups are analyzed by U Mann-Whitney if the sample does not follow a normal distribution (significance value in the Kolmogorov-Smirnov normality test).
iii.Comparisons between groups are analyzed by Student’s t-test if the sample follows a normal distribution (no significant value in the Kolmogorov-Smirnov normality test). - A P-value < 0.05 is used to determine statistical significance.
Note: The example of graphs presented in this section are the data obtained comparing mice with a chemogenetic inhibition of PL-NAc core pathway and mice with no inhibition of this network. All this data is published in the manuscript Domingo-Rodriguez et al., 2020.
- Graphs: The data of the 3 criteria are expressed as individual values with the median and the interquartile range (Figure 11).
Notes
Bodyweight and food intake are measured once a week during the entire short and long food addiction mouse protocols. These measurements are especially crucial in the short food addiction mouse protocol in which it is used osmotic minipumps filled with CNO. We demonstrated that in our conditions, no side effects of CNO are revealed on body weight, food intake, and neither in locomotor activity (Domingo-Rodriguez et al., 2020).
Acknowledgments
We thank E. Senabre, S. Kummer for their critics and technical support. This work was supported by the Spanish Ministerio de Economía y Competitividad-MINECO (#SAF2017-84060-R-AEI/FEDER-UE), the Spanish Instituto de Salud Carlos III, RETICS-RTA (#RD12/0028/0023), the Generalitat de Catalunya, AGAUR (#2017 SGR-669), ICREA-Acadèmia (#2015) and the Spanish Ministerio de Sanidad, Servicios Sociales e Igualdad, Plan Nacional Sobre Drogas (#PNSD-2017I068) to R.M., Fundació La Marató-TV3 (#2016/20-30) and Plan Nacional Sobre Drogas of the Spanish Ministry of Health (#PNSD-2019I006) to E.M-G. The methodology described was previously used in Domingo-Rodriguez et al., 2020. Figures with drawings are created with BioRender.com.
Competing interests
The authors have no conflicts of interest.
Ethics
All experimental protocols were performed in accordance with the guidelines of the European Communities Council Directive 2010/63/EU and approved by the local ethical committee (Comitè Ètic d'Experimentació Animal-Parc de Recerca Biomèdica de Barcelona, CEEA-PRBB, agreement N 9687).
References
- Deroche-Gamonet, V., Belin, D. and Piazza, P. V. (2004). Evidence for addiction-like behavior in the rat. Science 305(5686): 1014-1017.
- Domingo-Rodriguez, L., Ruiz de Azua, I., Dominguez, E., Senabre, E., Serra, I., Kummer, S., Navandar, M., Baddenhausen, S., Hofmann, C., Andero, R., Gerber, S., Navarrete, M., Dierssen, M., Lutz, B., Martín-García, E. And Maldonado, R. (2020). A specific prelimbic-nucleus accumbens pathway controls resilience versus vulnerability to food addiction. Nat Commun 11(1): 782.
- Gearhardt, A. N., Corbin, W. R. and Brownell, K. D. (2016). Development of the Yale Food Addiction Scale Version 2.0. Psycho Addict Behav 30(1): 113-121.
- Hamer, D. (2002). Genetics. Rethinking behavior genetics. Science 298(5591): 71-72.
- Koob, G. F. and Volkow, N. D. (2010). Neurocircuitry of addiction. Neuropsychopharmacology 35(1): 217-238.
- Koob, G. F. and Volkow, N. D. (2016). Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry 3(8): 760-773.
- Lindgren, E., Gray, K., Miller, G., Tyler, R., Wiers, C. E., Volkow, N. D. and Wang, G. J. (2018). Food addiction: A common neurobiological mechanism with drug abuse. Front Biosci (Landmark Ed) 23: 811-836.
- Logan, G. D., Schachar, R. J. and Tannock, R. (1997). Impulsivity and Inhibitory Control. Psychol Sci 8(1): 60-64.
- Mancino, S., Burokas, A., Gutierrez-Cuesta, J., Gutierrez-Martos, M., Martin-Garcia, E., Pucci, M., Falconi, A., D'Addario, C., Maccarrone, M. and Maldonado, R. (2015). Epigenetic and Proteomic Expression Changes Promoted by Eating Addictive-Like Behavior. Neuropsychopharmacology 40(12): 2788-2800.
- Martin-Garcia, E., Burokas, A., Kostrzewa, E., Gieryk, A., Korostynski, M., Ziolkowska, B., Przewlocka, B., Przewlocki, R. and Maldonado, R. (2011). New operant model of reinstatement of food-seeking behavior in mice. Psychopharmacology (Berl) 215(1): 49-70.
- Moore, C. F., Sabino, V., Koob, G. F. and Cottone, P. (2017). Pathological Overeating: Emerging Evidence for a Compulsivity Construct. Neuropsychopharmacology 42(7): 1375-1389.
- Nestler, E. J., Pena, C. J., Kundakovic, M., Mitchell, A. and Akbarian, S. (2016). Epigenetic Basis of Mental Illness. Neuroscientist 22(5): 447-463.
- Piazza, P. V. and Deroche-Gamonet, V. (2013). A multistep general theory of transition to addiction. Psychopharmacology (Berl) 229(3): 387-413.
- Pursey, K. M., Stanwell, P., Gearhardt, A. N., Collins, C. E. and Burrows, T. L. (2014). The prevalence of food addiction as assessed by the Yale Food Addiction Scale: a systematic review. Nutrients 6(10): 4552-4590.
- Schoenbaum, G., Roesch, M. R., Stalnaker, T. A. and Takahashi, Y. K. (2011). Orbitofrontal Cortex and Outcome Expectancies: Optimizing Behavior and Sensory Perception. In: Gottfried, J. A. (Ed.). Neurobiology of Sensation and Reward. Boca Raton (FL): CRC Press/Taylor & Francis.
Article Information
Publication history
Accepted: Jul 30, 2020
Published: Oct 5, 2020
Copyright
© 2020 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Martín-García, E., Domingo-Rodriguez, L. and Maldonado, R. (2020). An Operant Conditioning Model Combined with a Chemogenetic Approach to Study the Neurobiology of Food Addiction in Mice. Bio-protocol 10(19): e3777. DOI: 10.21769/BioProtoc.3777.
Category
Neuroscience > Nervous system disorders > Animal model
Neuroscience > Behavioral neuroscience > Animal model
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Tips for asking effective questions
+ Description
Write a detailed description. Include all information that will help others answer your question including experimental processes, conditions, and relevant images.
Share
Bluesky
X
Copy link







