- Submit a Protocol
- Receive Our Alerts
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Preparation and Transfection of Populus tomentosa Mesophyll Protoplasts
Published: Vol 11, Iss 22, Nov 20, 2021 DOI: 10.21769/BioProtoc.4220 Views: 2785
Reviewed by: Wenrong HeYuan WangYe XuNicole Gibbs

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols
Enrichment of Cytoplasmic RNA Granules from Arabidopsis Seedlings
Zhen Lei [...] Jungnam Cho
Nov 5, 2021 2053 Views

Fractionation and Extraction of Crude Nuclear Proteins From Arabidopsis Seedlings
Jiajia Zhao [...] Feifei Xu
Jan 20, 2022 3528 Views
Isolation of Intact Vacuoles from Arabidopsis Root Protoplasts and Elemental Analysis
Chuanfeng Ju [...] Zhenqian Zhang
Mar 5, 2023 380 Views
Abstract
Mesophyll protoplasts freshly isolated from leaves are a useful research system in plants. However, cell walls in woody plants contain more pectin, making mesophyll protoplasts isolation difficult in Populus. This has limited their application in biochemical, molecular, cellular, genetic, genomic, transcriptomic, and proteomic assays. In this protocol, a simple and efficient method to prepare and transfect mesophyll protoplasts of Populus tomentosa is presented in detail. Leaves of P. tomentosa plants grown in tissue culture media were pre-treated in D-mannitol solution and then digested with an enzyme solution. After washing with W5 and MMg buffers, the protoplasts were incubated in PEG/Ca2+ solution with plasmid for transfection. The mesophyll protoplasts isolated were used to express the histone variant H2B fused with green fluorescent protein (GFP) for confocal microscopy imaging. This “P. tomentosa mesophyll protoplasts preparation and transfection” system provides a useful tool for studying woody plants using a variety of applications, including gene expression, subcellular localization, protein-protein interaction, chromatin immunoprecipitation, western blot, single-cell sequencing, and genome editing.
Keywords: PopulusBackground
As perennials species, Populus is an ideal model system to explore scientific questions that can not be easily addressed in annual plants such as Arabidopsis and rice (Jansson and Douglas, 2007). For instance, autumn leaf senescence (Wang et al., 2021), sex determination (Muller et al., 2020; Xue et al., 2020), wood formation (Chen et al., 2019), and seasonal dormancy (Tylewicz et al., 2018), can be more easily studied in Populus than in annual plants. As the lifespan of Populus plants usually spans decades, centuries, or even millennia (Munné-Bosch, 2008), it is necessary to establish a simple and rapid system to study gene function and signal transduction in woody plants. Mesophyll protoplasts are a powerful and versatile tool for conducting cell-based experiments to study gene function and signaling pathways (Yoo et al., 2007). They provide a good way to study important scientific questions at biochemical, molecular, cellular, genetic, genomic, transcriptomic, proteomic, and single-cell levels. Previously reported protocols for preparation and transfection of mesophyll protoplasts mainly focused on the model plant Arabidopsis (Yoo et al., 2007). However, the protocol for isolating Arabidopsis mesophyll protoplasts is not suitable for Populus protoplast preparation because of the different cell wall components (Lin et al., 2014). The protocol summarized here is a simple (fewer reagents, only four reagents are required) and efficient (less time, isolation and transfection takes approximately 7 h) procedure (Figure 1) compared with previously reported Populus protoplast preparation (Guo et al., 2012; Tan et al., 2013). A histone variant HTB9 fused with GFP is transiently expressed in P. tomentosa mesophyll protoplasts (Figure 2). This cellular system provides a helpful tool for studying gene expression in woody plants. Moreover, this protocol can also be applied for other Populus species leaves such as Populus alba, Populus trichocarpa, Populus alba × P. tremula var. glandulosa (84K), and Populus davidiana × P. bolleana, or leaves of other woody species such as Eucalyptus urophylla × E. grandis or Eucalyptus grandis × E. urophylla grown in tissue culture media.
Materials and Reagents
10 ml centrifugal tubes (HOUDIOR)
1.5 ml microfuge tubes (Axygen)
Blades (Gillette blue, Super Gillette Blue Blades, 4.3 cm × 2.2 cm)
Culture dish (90 mm diameter)
Glass-bottom dish (In Vitro Scientific, catalog number: D35-10-1.5-N)
Strainers, 400 mesh (BIODEE, catalog number: DE2009)
Cellulase “ONOZUKA” R-10 (Yakult, catalog number: L0012-10g)
Macerozyme R-10 (Yakult, catalog number: L0021-5g)
Pectolase Y-23 (Yakult, product agent (BIODEE) catalog number: AOV0094-1g)
MES (Sigma-Aldrich, catalog number: M3671-50G)
PEG4000 (Sigma-Aldrich, catalog number: 81240-1KG)
D-glucose (Sigma-Aldrich, catalog number: G7021-100G)
β-thioglycol (Amresco, catalog number: 0482-100 ml)
BSA (Amresco, catalog number: 0332-100g)
D-Mannitol (BIODEE, catalog number: BN20023-250g)
KCl (BIODEE, catalog number: DE-0395A-250g)
NaCl (BIODEE, catalog number: DE0008-500g)
MgCl2·6H2O (BIODEE, catalog number: DE-0288A-500g)
CaCl2 (BIODEE, catalog number: DE-0556A-500g)
Plasmid (dissolve in double distilled water, 2 µg/µl)
Enzymatic solution (see Recipes) (Table 1)
W5 solution (see Recipes) (Table 2)
MMg solution (see Recipes) (Table 3)
PEG/Ca2+ solution (see Recipes) (Table 4)
D-mannitol solution (see Recipes)
Equipment
1,000 µl micropipette (Eppendorf)
200 µl micropipette (Eppendorf)
10 ul micropipette (Eppendorf)
Cell counting plate (MARIENFELD, model: MARIENFELD-0650030)
Erlenmeyer flasks (Bomex, 50 ml, 500 ml)
Plant incubator (Percival, model: AR-36L3)
Bacterial shaker incubator (Crystal Technology & Industries, model: IS-AX-190L)
Centrifuge (Eppendorf, model: 5804 R)
Confocal microscope (Carl Zeiss, model: Zeiss LSM780)
Procedure
Preparation of P. tomentosa materials and solutions
One-month-old P. tomentosa young plants are grown in tissue culture media. Stem fragments are cultured on shoots propagation medium [Murashige and Skoog (MS) medium containing 0.1 mg/L α-naphthalene acetic acid (NAA), 0.02 mg/l thidiazuron (TDZ)] for 30 days to get plantlets, then the plantlets are transferred to rooting medium (1/2-strength MS containing 0.05 mg/L NAA) with a 16/8 h (light/dark) photoperiod. After 30 days, the plantlets are approximately 10 cm in height (Figure 1).
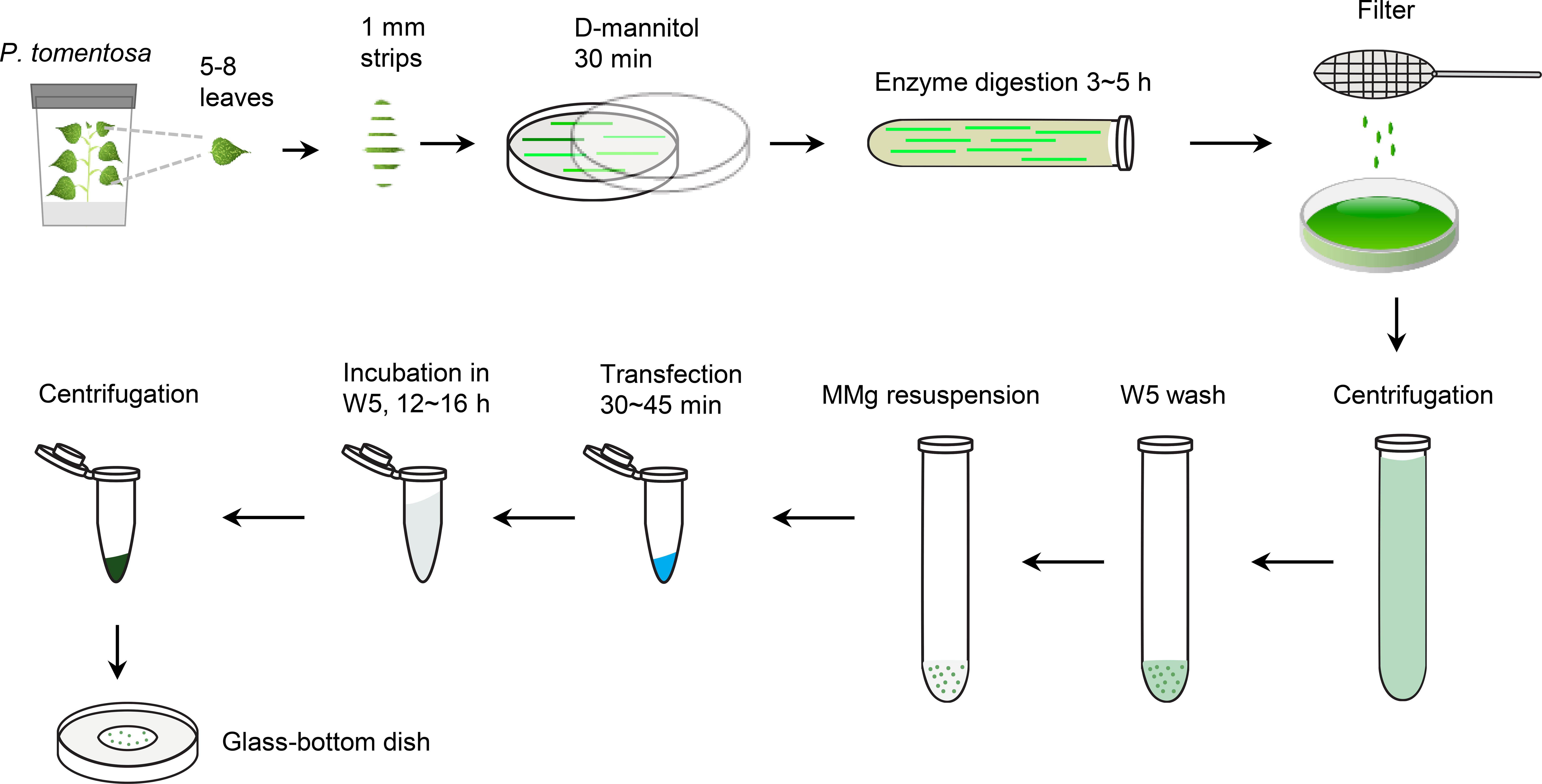
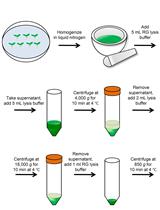
Figure 1. Experimental scheme of the preparation and transfection of P. tomentosa mesophyll protoplasts. Cut the leaves of one-month-old P. tomentosa plants grown on tissue culture media into 1 mm strips pre-treated in 0.8 M D-mannitol for 20 min. Digest the leaf strips in enzyme solution for 3-5 h, then filter with 400-mesh cell strainer, centrifuge and wash the protoplasts with W5 solution twice, followed by wash with MMg buffer twice. The transfection reaction containing 10 μl plasmid, MMg resuspended protoplasts (100 μl), and 40% PEG/Ca2+ solution (110 μl) occurs for 50 min. Wash with W5 solution and incubate for 12-16 h. Centrifuge and observe the transfected protoplasts using a glass-bottom dish.Prepare enzymatic, W5, MMg, and PEG/Ca2+ solutions. Place the W5 and MMg solutions at 4°C for at least 1 h before use.
Isolation of P. tomentosa protoplasts
Prepare D-mannitol solution (see Recipes).
Use a sharp blade to cut 5 P. tomentosa young leaves into 1 mm wide strips, completely immerse them in the D-mannitol solution, and place the culture dish on the bench (room temperature, 23°C) for 20 min.
Transfer the leaf strips into a 10 ml centrifuge tube with the prepared enzymatic solution, place the tube horizontally in the bacterial shaker incubator in the dark, and shake at 10 rpm for 3-5 h at 23°C until the color of solution changes to green.
Filter with a 400-mesh cell strainer, transfer the filtrate into a new 10 ml centrifuge tube, and centrifuge at 100 × g for 2 min at 23°C. Remove the supernatant and keep 2 ml of the precipitate.
Plasmid transfection
Add 2 ml of pre-cooled W5 solution gently along the tube wall to wash the protoplasts, gently invert and mix, centrifuge at 100 × g for 1 min at 4°C, and remove the supernatant.
Repeat C1 once.
Resuspend the protoplasts with 2 ml of pre-cooled W5 solution and place on ice for 30 min.
Centrifuge at 100 × g for 1 min at 4°C and remove the supernatant.
Add 2 ml pre-cooled MMg solution to resuspend the protoplasts, centrifuge at 100 × g for 1 min at 4°C, and remove the supernatant.
Resuspend the protoplasts to 1 × 105-5 × 105 per ml (determine their density with the cell counting plate) with pre-cooled MMg solution, and use a cut micropipette tip (0.5-1 cm) to add 100 µl of protoplast suspension for each plasmid of gene transfection.
Handle the following steps at 23°C. Add 10 µl of plasmid (2 µg/µl) for a target gene to a 1.5 ml microfuge tubes, and add 100 µl of protoplast suspension liquid and gently mix with micropipette. Add 110 µl (equal volume) of the PEG/Ca2+ solution and gently mix with a cut micropipette tip (0.5-1 cm).
Incubate in the dark at 23°C for 50 min (transfection step).
Add 440 µl (twice volume) of W5 solution, gently mix with a cut micropipette tip (0.5 cm), centrifuge at 100 × g for 1 min at 23°C, and remove the supernatant.
Add 100 µl of W5 solution, mix with cut micropipette tip (0.5-1 cm), centrifuge at 100 × g for 1 min at 23°C, and remove the supernatant.
Repeat C10 once.
Add 1 ml of W5 solution, mix with the 1,000 µl micropipette, and incubate in the dark at 23°C for 12-16 h.
Centrifuge at 100 × g for 1 min at 23°C and remove the supernatant. Transfer the precipitate onto a glass-bottom dish for confocal microscopy observation (50-80% transfection efficiency, Figure 1). The results of transfection for PtHTB9 and EgHTB9 are displayed in Figures 2 and 3, respectively.
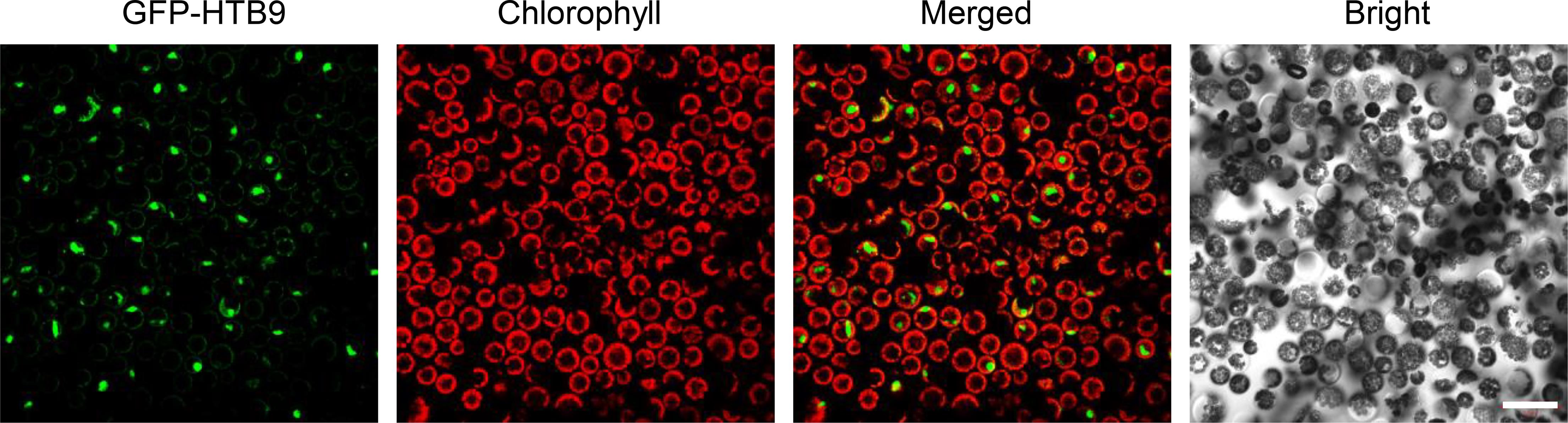
Figure 2. Transfection of GFP-PtHTB9 in P. tomentosa mesophyll protoplasts. The coding sequence (CDS) of the P. tomentosa HTB9 gene (PtHTB9, Potri.008G030600) was inserted into a modified pUC19-GFP vector, and 10 μl of GFP-HTB9 plasmid (2 μg/μl) was used for transfection. Images were taken with a Zeiss 780 confocal microscopy. Scale bar = 50 μm.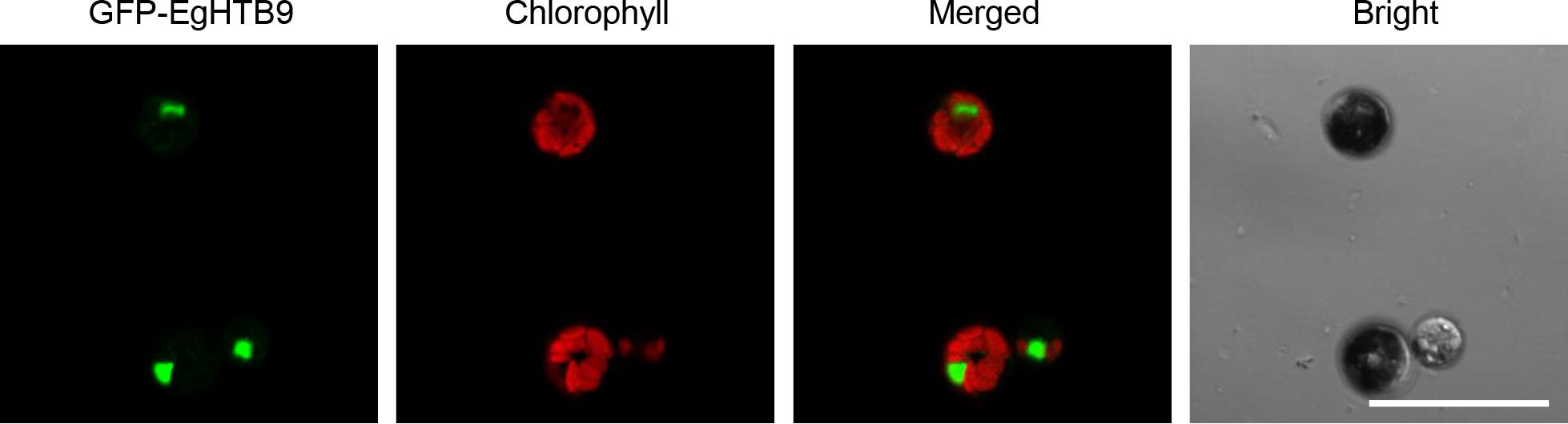
Figure 3. Transfection of GFP-EgHTB9 in Eucalyptus urophylla × E. grandis mesophyll protoplasts. The coding sequence (CDS) of the E. grandis HTB9 gene (EgHTB9, Eucgr.B03925) was inserted into a modified pUC19-GFP vector. Scale bar = 50 μm.
Notes
All the chemicals used in this study are Analytical Reagent (A.R.) standard.
For high transfection efficiency, we strongly recommend using PEG4000 (Sigma-Aldrich, catalog number: 81240-1KG) at a concentration of 40%.
Use scissors to cut off the tip (0.5-1 cm length) of all 100 µl micropipette tips for C6, C7, C10, and C11 steps, and cut off the tip (0.5 cm length) of 1,000 µl micropipette tips for C9 step.
Addition of β-mercaptoethanol to the enzymatic solution (Table 1) is optional.
Sterilized and separately stored W5 or MMg solutions can be placed at 4°C for 1 year, while enzymatic and PEG/Ca2+ solutions should be freshly prepared before use.
Set all the acceleration and deacceleration of centrifugation to 5 (max 10).
Leaves digested horizontally in 10 ml centrifuge tubes with 10 ml enzymatic solution provides better isolation. Too much air in the tubes (10 ml enzymatic solution in 15 ml centrifuge tubes) or higher speeds (>60 rpm) in step B3 lead to more broken protoplasts.
Smaller protoplast concentrations in the C6 step give higher transfection efficiency, while higher suspension concentration provides more yield (transfected cells).
Recipes
Enzymatic solution (Table 1)
Table 1. Recipe for the enzymatic solutionStock Solutions Weight or volume Final Concentration Cellulase R-10 0.15 g 1.5% Macerozyme R-10 0.04 g 0.4% Pectolase Y-23 0.05 g 0.5% D-mannitol 1.092 g 0.6 M 200 mM KCl 1 ml 20 mM 200 mM MES 1 ml 20 mM 5 ml ddH2O, vortex, 55°C 10 min, leave to cool at room temperature 1 M CaCl2 100 μl 10 mM 7.5 mM β-thioglycol 66.67 μl 0.05 mM 1% BSA 1 ml 0.1% Fill the volume to 10 ml with ddH2O W5 solution (Table 2)
Table 2. Recipe for the W5 solution
Stock Solutions Weight or volume Final Concentration 2 M NaCl 38 ml 154 mM 1 M CaCl2 62.5 ml 125 mM 200 mM KCl 12.5 ml 5 mM 200 mM MES 5 ml 2 mM D-glucose 0.45 g 5 mM Fill the volume to 500 ml with ddH2O MMg solution (Table 3)
Table 3. Recipe for the MMg solution
Stock Solutions Weight or volume Final Concentration D-mannitol 7.29 g 0.6 M 150 mM MgCl2 10 ml 15 mM 200 mM MES 2 ml 4 mM Fill the volume to 100 ml with ddH2O PEG/Ca2+ solution (Table 4)
Table 4. Recipe for the PEG/Ca2+ solutionStock Solutions Weight or volume PEG4000 3 g ddH2O 2.25 ml 0.8 M D-mannitol 1.785 ml 1 M CaCl2 0.75 ml D-mannitol solution
Dissolve 2.912 g mannitol in 20 ml ddH2O in 90 mm diameter culture dish.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (31900173 to H.-L.W.; 31970196 to Z.L.; 31770649 to X.X.). This protocol was modified from previous work (Yoo et al., 2007; Guo et al., 2012; Tan et al., 2013; Lin et al., 2014), and we would like to thank all who contributed to these previously published procedures.
Competing interests
No financial, personal, or professional interests have influenced the work.
References
- Chen, H., Wang, J. P., Liu, H., Li, H., Lin, Y. J., Shi, R., Yang, C., Gao, J., Zhou, C. and Li, Q. (2019). Hierarchical transcription factor and chromatin binding network for wood formation in black cottonwood (Populus trichocarpa). Plant Cell 31(3): 602-626.
- Guo, J., Morrell-Falvey, J. L., Labbe, J. L., Muchero, W., Kalluri, U. C., Tuskan, G. A. and Chen, J. G. (2012). Highly efficient isolation of Populus mesophyll protoplasts and its application in transient expression assays. PLoS One 7(9): e44908.
- Jansson, S. and Douglas, C. J. (2007). Populus: a model system for plant biology. Annu Rev Plant Biol 58435-458.
- Lin, Y. C., Li, W., Chen, H., Li, Q., Sun, Y. H., Shi, R., Lin, C. Y., Wang, J. P., Chen, H. C., Chuang, L., Qu, G. Z., Sederoff, R. R. and Chiang, V. L. (2014). A simple improved-throughput xylem protoplast system for studying wood formation. Nat Protoc 9(9): 2194-2205.
- Muller, N. A., Kersten, B., Leite Montalvao, A. P., Mahler, N., Bernhardsson, C., Brautigam, K., Carracedo Lorenzo, Z., Hoenicka, H., Kumar, V. and Mader, M. (2020). A single gene underlies the dynamic evolution of poplar sex determination. Nat Plants 6(6): 630-637.
- Munné-Bosch, S., (2008). Do perennials really senesce. Trends Plant Sci 13(5): 216-220.
- Tan, B., Xu, M., Chen, Y. and Huang, M. (2013). Transient expression for functional gene analysis using Populus protoplasts. Plant Cell, Tissue Organ Cult 114(1): 11-18.
- Tylewicz, S., Petterle, A., Marttila, S. and Miskolczi, P. (2018). Photoperiodic control of seasonal growth is mediated by ABA acting on cell-cell communication. Science 360(6385): 212-215.
- Wang, H. L., Zhang, Y., Wang, T., Yang, Q., Yang, Y., Li, Z., Li, B., Wen, X., Li, W., Yin, W., Xia, X., Guo, H. and Li, Z. (2021). An alternative splicing variant of PtRD26 delays leaf senescence by regulating multiple NAC transcription factors in Populus. Plant Cell 33(5): 1594-1614.
- Xue, L., Wu, H., Chen, Y., Li, X., Hou, J., Lu, J., Wei, S., Dai, X., Olson, M. S., Liu, J., Wang, M., Charlesworth, D. and Yin, T. (2020). Evidences for a role of two Y-specific genes in sex determination in Populus deltoides. Nat Commun 11(1): 5893.
- Yoo, S. D., Cho, Y. H. and Sheen, J. (2007). Arabidopsis mesophyll protoplasts: a versatile cell system for transient gene expression analysis. Nat Protoc 2(7): 1565-1572.
Article Information
Publication history
Accepted: Aug 13, 2021
Published: Nov 20, 2021
Copyright
© 2021 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Wang, H. L., Wang, T., Yang, Q., Yin, W., Xia, X., Guo, H. and Li, Z. (2021). Preparation and Transfection of Populus tomentosa Mesophyll Protoplasts. Bio-protocol 11(22): e4220. DOI: 10.21769/BioProtoc.4220.
Category
Plant Science > Plant cell biology > Organelle isolation
Biological Sciences > Biological techniques
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Tips for asking effective questions
+ Description
Write a detailed description. Include all information that will help others answer your question including experimental processes, conditions, and relevant images.
Share
Bluesky
X
Copy link