- Submit a Protocol
- Receive Our Alerts
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
A Method to Induce Brown/Beige Adipocyte Differentiation from Murine Preadipocytes
Published: Vol 11, Iss 24, Dec 20, 2021 DOI: 10.21769/BioProtoc.4265 Views: 2902
Reviewed by: Ralph Thomas BoettcherClara Lubeseder-MartellatoKossay ZaouiAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols
Spatial Centrosome Proteomic Profiling of Human iPSC-derived Neural Cells
Fatma Uzbas and Adam C. O’Neill
Sep 5, 2023 458 Views
Preparation of Cardiac Extracts from Embryonal Hearts to Capture RNA–protein Interactions by CLIP
Giulia Buonaiuto [...] Monica Ballarino
Oct 20, 2023 317 Views
Generation of Human Blood Vessel and Vascularized Cerebral Organoids
Xin-Yao Sun [...] Zhen-Ge Luo
Nov 5, 2023 475 Views
Abstract
Adipocytes exhibit different morphological and functional characteristics, depending on their anatomical location, developmental origin, and stimulus. While white adipocytes tend to accumulate energy as triglycerides, brown and beige adipocytes tend to direct carbon sources to fuel thermogenesis. White and beige adipocytes originate from common progenitor cells, which are distinct from brown adipocyte precursors. Having a method to study white vs. beige vs. brown adipocyte differentiation may help to unveil the mechanisms driving distinct adipogenic programs. Preadipocytes can be cultured and differentiated in vitro using a combination of compounds to stimulate adipogenesis. Here, we describe and compare protocols designed to stimulate adipocyte differentiation and induce brown/beige-like or white-like characteristics in differentiating adipocytes. The protocols consist in exposing murine preadipocytes to pharmacological stimuli aimed at triggering adipogenesis and inducing (or not) a thermogenic gene expression program. After 8 days of differentiation with a pro-browning cocktail, immortalized preadipocytes isolated from interscapular brown fat (9B cells) or inguinal white fat (9W cells) from the same mouse expressed higher levels of brown/beige adipocyte markers (e.g., Ucp1) and pan-adipocyte differentiation markers (e.g., Pparg, Cebpa and aP2) when compared to the same cells differentiated with a cocktail that lacked brown/beige adipogenic inducers (i.e., rosiglitazone, T3, and indomethacin). Consistent with a higher thermogenic potential of brown vs. beige adipocytes, differentiated 9B cells expressed higher Ucp1 levels than differentiated 9W cells. This simple protocol may help researchers to understand mechanisms of adipogenesis and how adipocytes become thermogenic.
Background
Obesity is characterized by excess body fat and represents a risk factor for several chronic non-transmissible diseases (World Health Organization, 2015). The activation of brown adipose tissue (BAT) and the recruitment of newly formed beige adipocytes in white adipose tissue (WAT) leads to increased thermogenesis and higher energy expenditure, thus representing a promising intervention to treat obesity and its complications. Brown adipocytes contain multiple lipid droplets in their cytoplasm. They are also filled with mitochondria that promote energy dissipation in the form of heat, by leaking protons across the inner mitochondrial membrane, thus uncoupling oxidative phosphorylation from ATP synthesis. This mechanism is mediated by uncoupling protein 1 (UCP1), in a process called non-shivering thermogenesis (Nedergaard et al., 2001; Cannon and Nedergaard, 2004). White adipocytes have a single and large lipid droplet, few mitochondria, and produce many types of adipokines (Rosen and Spiegelman, 2014). Adipocytes with increased thermogenic capacity also appear in WAT depots, under states such as cold exposure, exercise, and caloric restriction (Cinti et al., 2005; Wu et al., 2012; Fabbiano et al., 2016), and are called beige adipocytes. Although beige adipocytes are functionally similar to brown adipocytes, these two types of thermogenic cells differ in their developmental origin (Ikeda et al., 2018). In fact, beige adipocytes share common progenitor cells with white adipocytes (Ikeda et al., 2018). The process of beige adipogenesis is often called browning, and normally occurs in subcutaneous fat depots. Together, these observations suggest that cell extrinsic and intrinsic factors contribute to determine the fate of adipocyte differentiation. Understanding these factors may help elucidating adipogenesis and lead to the identification of new therapeutic targets (Vegiopoulos et al., 2017). Here, we used murine preadipocyte cell lines isolated from different fat depots to characterize and compare protocols to promote brown, beige, or white adipocyte differentiation. These protocols were based on previous studies (Morrison and Farmer, 1999; Fasshauer et al., 2001; Tseng et al., 2004), although they have been slightly modified in our laboratory, where they have been replicated and optimized to apply basic procedures, budget-friendly reagents, and minimal infrastructure.
Materials and Reagents
12-well Cell Culture Plate (Corning, 12-565-321)
0.22 μm sterile filter
9B preadipocytes: these cells were isolated from the interscapular brown fat of a Dicerlox/lox; aP2-Cre-ERT2 mouse and immortalized using SV-40-large T antigen as described before (Mori et al., 2012 and 2014). They behave like wild type cells unless 4-hydroxytamoxifen is added to the medium
9W preadipocytes: these cells were isolated from the subcutaneous white fat of the same mouse used to isolate 9B cells. These cells were immortalized using SV-40-large T antigen and also behave like wild type cells unless 4-hydroxytamoxifen is added to the medium
20 nM insulin* (Sigma-Aldrich, catalog number: I6634, powder storage temperature -20°C. Store at 4°C after dilution)
1 nM triiodothyronine (T3)* (Sigma-Aldrich, catalog number: T2877, storage temperature -20°C)
0.5 mM 3-Isobutyl-1-methylxanthine (IBMX)* (Sigma-Aldrich, catalog number: I5879, storage temperature -20°C)
1 μM dexamethasone* (Sigma-Aldrich, catalog number: D4902, storage temperature -20°C)
0.125 mM indomethacin* (Sigma-Aldrich, catalog number: I7378, storage temperature -20°C)
2.8 μM rosiglitazone* (Sigma-Aldrich, catalog number: R2408, storage temperature -20°C)
Fetal bovine serum (FBS) (Thermo Fisher Scientific, catalog number: 12657-029, storage temperature -20°C)
Penicillin-Streptomycin (Pen/Strep) (Thermo Fisher Scientific, catalog number: 15140122, storage temperature -20°C)
Dulbecco’s modified Eagle’s medium (DMEM), high glucose (Life Technologies, InvitrogenTM, catalog number: 11965, storage temperature 4°C)
1 mM insulin stock solution (see Recipes)
10 μM T3 stock solution (see Recipes)
0.25 M IBMX stock solution (see Recipes)
1 mM dexamethasone stock solution (see Recipes)
0.125 M indomethacin stock solution (see Recipes)
2.8 mM rosiglitazone stock solution (see Recipes)
Maintenance medium (see Recipes)
Differentiation medium day 2 (see Recipes)
Differentiation medium day 4 (see Recipes)
Differentiation medium day 6 (see Recipes)
*Final concentration
Equipment
Heating block
Tissue culture incubator (37°C, 5% CO2, Sheldon Manufacturing, Inc.)
Water bath (Fisher Scientific, FS-205)
Software
Graphpad Prism 9® software
Procedure
Note: Pre-warm media (see Recipes) to 37°C for 10 min prior to the procedure.
Seed cells
Seed 1 × 105 preadipocytes per well in a 12-well plate and culture in 1 ml of “Maintenance medium” (see Recipes: B1 for pro-browning induction, or C1 for pro-whitening).
Change media every 2 days until 90% confluence.
Adipocyte differentiation
Day 0: Once cells reach 90% confluence, change media and culture for 2 days with “Maintenance medium”.
Day 2: Replace media with 1 ml of “Differentiation medium day 2” (see Recipes: B2 for pro-browning, or C2 for pro-whitening) and culture for 2 days.
Day 4: Remove media and replace with 1 ml of “Differentiation medium day 4” (see Recipes: B3 for pro-browning, or C3 for pro-whitening).
Day 6: After 2 days, replace the media with 1 ml of “Differentiation medium day 6” (see Recipes: B4 for pro-browning, or C4 for pro-whitening).
Day 8: Cells are fully differentiated and ready to use.
Data analysis
Validation
Here, we describe a method to induce adipocyte differentiation in vitro from murine preadipocytes. We describe two types of differentiation protocols: one that leads to the induction of a brown/beige adipogenic profile (“Pro-Browning Cocktail Recipe”) and another that leads to differentiation of white adipocytes (“Pro-Whitening Cocktail Recipe”). In addition to the cells described here, we have applied these protocols in a wide range of murine preadipocyte cell lines, including 3T3-F442A, C3H10T1/2, and others (data not shown and Rocha et al., 2020). The protocols lead to 50-90% of cells exhibiting lipid droplets in their cytoplasm, although the proportion of differentiated cells and the size of the lipid droplets will largely depend on a number of factors, including the lot of FBS, and the type, passage, commitment, and confluency of the preadipocytes. To illustrate the protocol’s efficiency, we used immortalized preadipocytes isolated from the subcutaneous white adipose tissue (9W cells) and brown adipose tissue (9B cells) of the same mouse and differentiated them with the Pro-Browning Cocktail Recipe or the Pro-Whitening Cocktail Recipe. The Pro-Browning Cocktail Recipe induced the expression of Ucp1 in both cell lines, although the level of induction was higher in 9B cells by several orders of magnitude (Figure 1A). Additionally, it further induced pan-adipocyte markers Cebpa (Figure 1B), Pparg (Figure 1C), and aP2 (Figure 1D) when compared to the Pro-Whitening Cocktail Recipe. These results indicate that not only 9B preadipocytes are more committed to adipogenesis, but also that the Pro-Browning Cocktail is more potent than the Pro-Whitening Cocktail to induce adipocyte differentiation.
Adipocytes differentiated using the procedures described here can be used in several different applications. In our original research paper where the Pro-Browning Cocktail protocol was applied (Rocha et al., 2020), we performed multiple experimental procedures using differentiated adipocytes, including gene expression analysis, measurement of oxygen consumption, and confocal and fluorescence lifetime imaging microscopy. Brown/beige adipocyte-like features were observed in 9W cells differentiated with the Pro-Browning Cocktail protocol. These features included the expression of a thermogenic gene profile, increased mitochondrial content, multilocular lipid accumulation, elevated oxygen consumption rate with high uncoupled respiration, and reduced NADH and FAD lifetime. All data and information about data processing and analysis, including statistics and details of replicates, are included in the original research paper (Rocha et al., 2020).
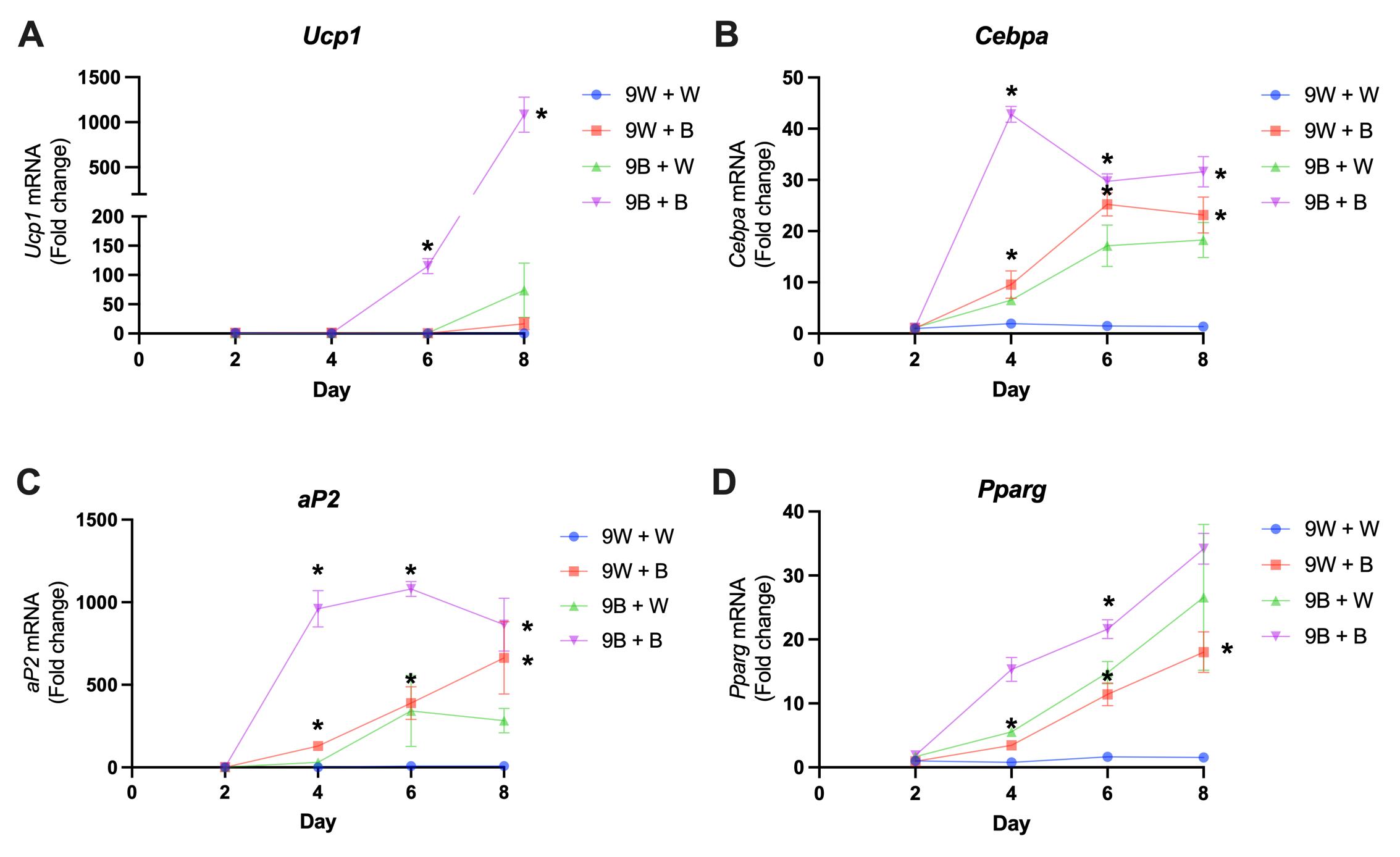
Figure 1. Comparison between the pro-browning (B) and pro-whitening (W) cocktails to induce adipocyte differentiation using murine immortalized white (9W) or brown (9B) preadipocytes. White (9W) and brown (9B) preadipocytes were differentiated using the pro-whitening (9W + W and 9B + W), or the pro-browning (9W + B and 9B + B) cocktails. Expression of (A) Ucp1, (B) Cebpa, (C) aP2, and (D) Pparg mRNA was assessed by real-time qPCR (n = 3 independent pools of cells per group). 36B4 mRNA expression was used for normalization. Values are the mean ± SEM.*P < 0.05 versus the pro-whitening cocktail (W) (One-way ANOVA with Tukey's multiple comparison test). Primer sequences are available upon request.
Statistical analysis
The results were expressed as mean ± standard error of the mean (SEM) and analyzed statistically by the One-way ANOVA with Tukey's multiple comparison test, adopting a minimum significance limit of P < 0.05 in the Graphpad Prism 7® software.
Recipes
Preparation of stock solutions
1 mM insulin stock solution in 1 ml of distilled water (add 0.5 µl HCl 16 N for better solubilization). Filter the stock solution through a 0.22 μm sterile filter.
10 μM T3 stock solution in ethanol
0.25 M IBMX stock solution in 0.5 M KOH. Filter the stock solution through a 0.22 μm sterile filter
1 mM dexamethasone stock solution in ethanol
0.125 M indomethacin stock solution in ethanol
2.8 mM rosiglitazone stock solution in ethanol
Notes:
Stocks may be stored for several months at -20°C, except for insulin, which needs to be stored at 4°C.
To help with solubilization, (3) and (5) may be heated to 75°C for 1 min using a heating block.
Pro-Browning Cocktail Recipe
Maintenance medium
DMEM high glucose supplemented with 10% FBS and 1% of Pen/Strep
Differentiation medium day 2
DMEM high glucose supplemented with 20 nM insulin, 1 μM dexamethasone, 0.5 mM IBMX, 1 nM T3, 0.125 mM indomethacin, and 2.8 μM rosiglitazone.
Differentiation medium day 4
DMEM high glucose supplemented with 20 nM insulin, 1 nM T3, and 2.8 μM rosiglitazone.
Differentiation medium day 6
DMEM high glucose supplemented with 20 nM insulin, and 2.8 μM rosiglitazone.
Pro-Whitening Cocktail Recipe
Maintenance medium
DMEM high glucose supplemented with 10% FBS and 1% of Pen/Strep.
Differentiation medium day 2
DMEM high glucose supplemented with 20 nM insulin, 1 μM dexamethasone, and 0.5 mM IBMX.
Differentiation medium day 4
DMEM high glucose supplemented with 20 nM insulin.
Differentiation medium day 6
DMEM high glucose supplemented with 20 nM insulin.
Acknowledgments
We are supported by grants from FAPESP (2017/01184-9, 2016/12294-7, 2012/07259-7 and 2015/03292-8), CNPq (474397/2011-4 and 305069/2015-2), and CAPES (1431744/2014-2016). This protocol was described and is linked to Rocha et al. (2020; DOI: 10.1126/sciadv.abc6250).
Competing interests
The authors declare no competing interests.
References
- Cannon, B. and Nedergaard, J. (2004). Brown adipose tissue: function and physiological significance. Physiol Rev 84(1): 277-359.
- Cinti, S., Mitchell, G., Barbatelli, G., Murano, I., Ceresi, E., Faloia, E., Wang, S., Fortier, M., Greenberg, A. S. and Obin, M. S. (2005). Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res 46(11): 2347-2355.
- Fabbiano, S., Suárez-Zamorano, N., Rigo, D., Veyrat-Durebex, C., Stevanovic Dokic, A., Colin, D. J. and Trajkovski, M. (2016). Caloric Restriction Leads to Browning of White Adipose Tissue through Type 2 Immune Signaling. Cell Metab 24(3): 434-446.
- Fasshauer, M., Klein, J., Kriauciunas, K. M., Ueki, K., Benito, M. and Kahn, C. R. (2001). Essential role of insulin receptor substrate 1 in differentiation of brown adipocytes. Mol Cell Biol 21(1): 319-329.
- Ikeda, K., Maretich, P. and Kajimura, S. (2018). The Common and Distinct Features of Brown and Beige Adipocytes. Trends Endocrinol Metab 29(3):191-200.
- Mori, M. A., Raghavan, P., Thomou, T., Boucher, J., Robida-Stubbs, S., Macotela, Y., Russell, S. J., Kirkland, J. L., Blackwell, T. K. and Kahn, C. R. (2012). Role of microRNA processing in adipose tissue in stress defense and longevity. Cell Metab 16(3): 336-347.
- Mori, M. A., Thomou, T., Boucher, J., Lee, K. Y., Lallukka, S., Kim, J. K., Torriani, M., Yki-Jarvinen, H., Grinspoon, S. K., Cypess, A. M. et al. (2014). Altered miRNA processing disrupts brown/white adipocyte determination and associates with lipodystrophy. J Clin Invest 124(8): 3339-3351.
- Morrison, R. F. and Farmer, S. R. (1999). Role of PPARgamma in regulating a cascade expression of cyclin-dependent kinase inhibitors, p18(INK4c) and p21(Waf1/Cip1), during adipogenesis. J Biol Chem 274(24): 17088-17097.
- Nedergaard, J., Golozoubova, V., Matthias, A., Asadi, A., Jacobsson, A. and Cannon, B. (2001). UCP1: the only protein able to mediate adaptive non-shivering thermogenesis and metabolic inefficiency. Biochim Biophys Acta 1504(1): 82-106.
- Rocha, A. L., de Lima, T. I., de Souza, G. P., Correa, R. O., Ferrucci, D. L., Rodrigues, B., Lopes-Ramos, C., Nilsson, D., Knittel, T. L., Castro, P. R., et al. (2020). Enoxacin induces oxidative metabolism and mitigates obesity by regulating adipose tissue miRNA expression. Sci Adv 6(49): eabc6250.
- Rosen, E. D. and Spiegelman, B. M. (2014). What we talk about when we talk about fat. Cell 156, 20-44.
- Tseng, Y. H., Kriauciunas, K. M., Kokkotou, E. and Kahn, C. R. (2004). Differential roles of insulin receptor substrates in brown adipocyte differentiation. Mol Cell Biol 24(5): 1918-1929.
- Vegiopoulos, A., Rohm, M. and Herzig, S. (2017). Adipose tissue: between the extremes. 36(14): 1999-2017.
- World Health Organization. (2015). Obesity and overweight. Report Geneva 311:1.
- Wu, J., Bostrom, P., Sparks, L. M., Ye, L., Choi, J. H., Giang, A. H., Khandekar, M., Virtanen, K. A., Nuutila, P. and Schaart, G. (2012). Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 150(2): 366-376.
Article Information
Publication history
Accepted: Oct 1, 2021
Published: Dec 20, 2021
Copyright
© 2021 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Rocha, A. L., Guerra, B. A., Boucher, J. and Mori, M. A. (2021). A Method to Induce Brown/Beige Adipocyte Differentiation from Murine Preadipocytes. Bio-protocol 11(24): e4265. DOI: 10.21769/BioProtoc.4265.
- Rocha, A. L., de Lima, T. I., de Souza, G. P., Correa, R. O., Ferrucci, D. L., Rodrigues, B., Lopes-Ramos, C., Nilsson, D., Knittel, T. L., Castro, P. R., et al. (2020). Enoxacin induces oxidative metabolism and mitigates obesity by regulating adipose tissue miRNA expression. Sci Adv 6(49): eabc6250.
Category
Developmental Biology > Cell growth and fate > Differentiation
Cell Biology > Cell isolation and culture > Cell differentiation
Biological Sciences > Biological techniques
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Tips for asking effective questions
+ Description
Write a detailed description. Include all information that will help others answer your question including experimental processes, conditions, and relevant images.
Share
Bluesky
X
Copy link