- Submit a Protocol
- Receive Our Alerts
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
A Behavioural Assay to Investigate Judgment Bias in Zebrafish
Published: Vol 12, Iss 4, Feb 20, 2022 DOI: 10.21769/BioProtoc.4327 Views: 1615
Reviewed by: Oneil Girish BhalalaNeloy Kumar Chakroborty Narayan Subramanian

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols
Measuring Behavioral Individuality in the Acoustic Startle Behavior in Zebrafish
Carlos Pantoja [...] Ehud Y. Isacoff
Apr 5, 2017 9043 Views

Anticipatory and Consummatory Responses to Touch and Food Rewards: A Protocol for Human Research
Emilio Chiappini [...] Sebastian Korb
Feb 20, 2022 1192 Views
A New Behavioral Paradigm for Visual Classical Conditioning in Drosophila
Mercedes Bengochea [...] Bassem Hassan
Nov 5, 2023 307 Views
Abstract
In this protocol, we describe for the first time a judgment bias paradigm to phenotype the way zebrafish assess ambiguous stimuli. We have developed and validated a protocol for a judgment bias test based on a Go/No-go task, and performed using a half radial maze. After a habituation phase, fish are trained to discriminate between two reference arms [positive (P) and negative (N)]. For this purpose, they experience a positive event (food reward in P), when presented with a specific location/color cue, and a negative event (chasing with net in N), when presented with a different location/color cue. Acquisition of the discrimination learning between P and N is revealed by the latencies to enter the experimental arms of the behavioral maze being significantly lower for the P arm than for the N arm. Once zebrafish are able to discriminate between P and N arms, their latency to enter other maze arms spatially located between P and N [(Near Positive (NP), Ambiguous (A) = half-way between P and N, and Near Negative (NN)] is analyzed. Latencies (L) to enter NP, A and NN maze arms are interpreted as indicating the individual expectancy to experience a reward/punishment on each of them. A judgment bias score (JBS) is calculated from the latencies to enter the P, N, and A arms for each fish [JBS = (LA–LP)*100/(LN–LP)], based on which fish can be classified into an optimistic/pessimistic axis. A JBS below 50 indicates that fish perceive the ambiguous stimulus as a positive one (optimistic bias), while JBS above 50 indicates that fish perceive the ambiguous stimulus as a negative one (pessimistic bias). However, for classification criteria, it could be advantageous to use the method of selecting extreme phenotypes (e.g., upper and lower quartiles of the JBS), since JBS in zebrafish falls into a bimodal distribution (unpublished data). Therefore, this protocol provides a unique, inexpensive, and effective alternative to other methods of measuring affective states in zebrafish that might be of great interest to a broad target audience and have a large number of applications.
Graphic abstract:
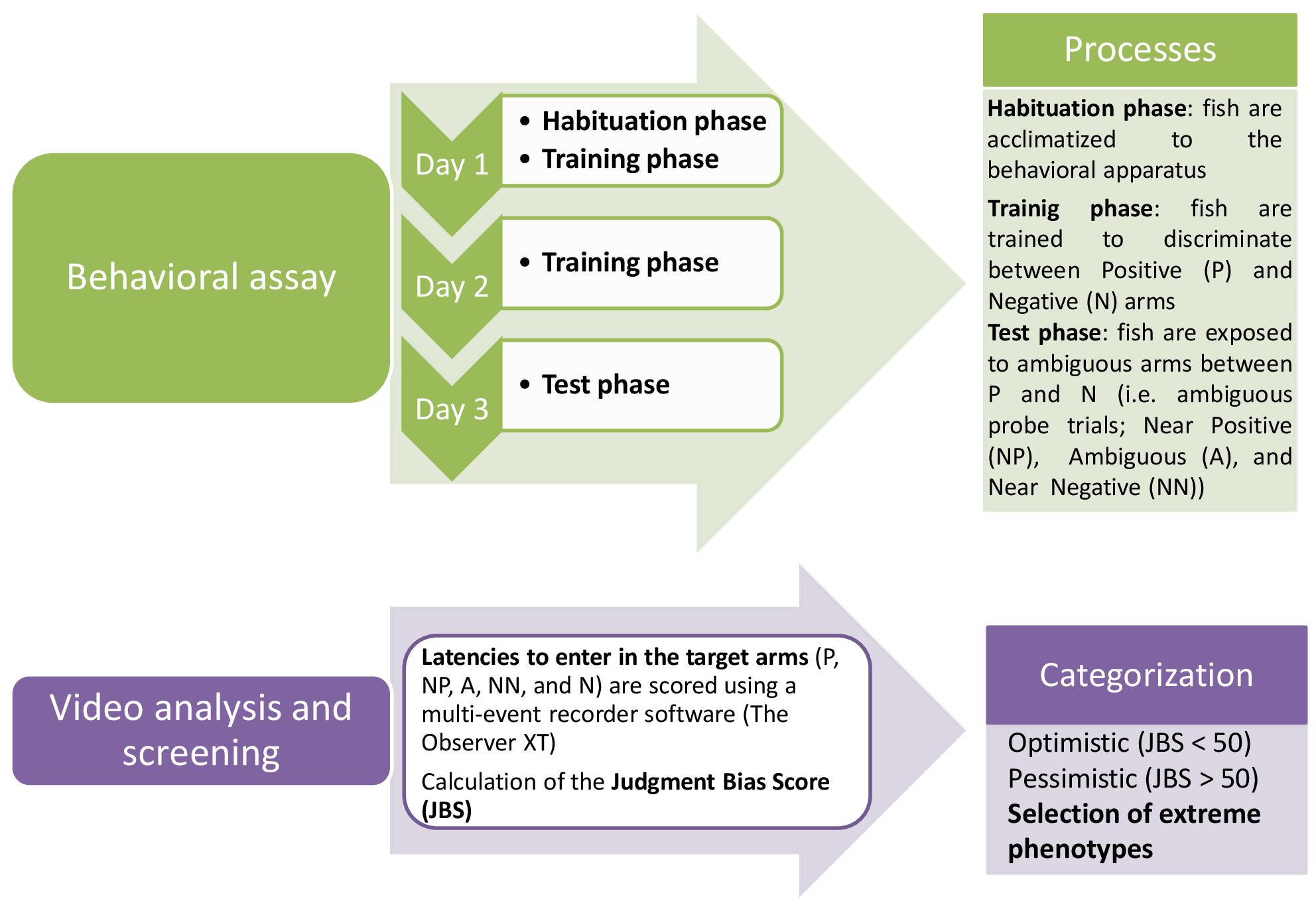
Flow chart of the judgment bias protocol in zebrafish.
Background
Zebrafish (Danio rerio) has emerged as a model organism for translational research in biomedicine, contributing to hundreds of studies on disease mechanisms, ranging from neurological and psychiatric disorders (Stewart et al., 2014), to cancer (White et al., 2013), and other age-associated diseases (Carneiro et al., 2016). Due to its robust phenotypes, and physiological (neuroanatomical, neuroendocrine, neurochemical) and genetic homologies to mammals, zebrafish has been considered as an ideal model for high-throughput behavioral assays for neurophenotyping and screening genetic mutations and psychotropic drugs. Here, we aim to describe a behavioral assay to measure judgment bias in zebrafish, in which some individuals consistently evaluate an ambiguous stimulus as negative (aka pessimists) and others as positive (aka optimists). Our assay is based on a judgment bias experimental paradigm originally developed for rodents (Harding et al., 2004), which was designed to measure expectations of positive (P) or negative (N) outcomes, when individuals are exposed to an ambiguous stimulus (A) that is intermediate between the two reference stimuli (P and N), and that they have learned to discriminate. After almost 20 years from its first publication, experimental tests to assess judgment bias have been developed and validated for a broad range of species (e.g., Mendl et al., 2010; Enkel et al., 2010; Bateson et al., 2011; Salmeto et al., 2011; Murphy et al., 2013). Most of the judgment bias studies have been focused on the effects of specific conditions inherent to life in captivity that are expected to impact the animal’s affective states. For instance, the effects of providing environmental enrichment (Douglas et al., 2012; Bethell and Koyama, 2015) and common handling procedures (Neave et al., 2013) have been broadly studied. Notably, the majority of judgment bias tasks have been shown to be very sensitive to manipulations that induce negative states (Baciadonna and McElligott, 2015). Furthermore, this behavioral paradigm has been used as a tool for assessing affective states in translational studies (Boleij et al., 2012; Rygula et al., 2015), reflecting the importance of judgment bias tests for biomedical research. However, the zebrafish have been unable to successfully perform judgment bias tasks, in large part due to their difficulties in categorizing more than one stimulus simultaneously. In fact, a number of different experimental designs based on Go/Go or Go/No-go tasks have already been tested in this species, in which the difficulties involved in selecting appropriate stimuli, balanced reward and punishment, and, importantly, in the simultaneous categorization of more than one stimulus were highlighted (Tan, 2017). Consequently, none of these assays was sufficiently reliable for judgment bias testing. In order to overcome these issues, we have used a combination of different stimulus classes (i.e., spatial and visual cues), which has been successfully operated in bumblebees (Perry et al., 2016) and may facilitate the acquisition of the relevant information for the performance of the task.
We believe the implications of the protocol reported here are of great significance for a broad variety of research fields due to its high applicability across research topics. The emerging evidence in the literature indicates that cognitive bias, and specifically judgment bias tasks, show promise as new measures of affective states in animals. Given the inaccessibility of direct reports of emotions in animals, an accurate assessment of their affective states is an important goal in animal welfare science. We also believe that this protocol is of general interest for translational scientists, with special mention of the research in psychiatric disorders, cancer, and other age-associated diseases, since it may provide an insight for inter-individual variation in susceptibility/resilience to these diseases. Considering the results of our Supporting Primary Paper (Espigares et al., 2021), we also believe that this protocol has the potential to be useful in the study of the in vivo efficacy of psychotropic drugs that have already been shown to exert a counteractive effect on telomere shortening. This is an important issue, since the correlation between the effect of telomerase activity by psychotropic medications (e.g., lithium and antidepressants) and their clinical efficacy still requires further investigation. On the other hand, a large number of psychological and neurophysiological studies suggest that judgments of ambiguous stimuli are the end result of a decision-making process that involves several components: sensory registration of the stimulus; evaluation of the stimulus and likely decision outcome; selection of a response. Therefore, the study of the cognitive and neural processes underlying judgment biases may also constitute a target for behavioral neuroscientists. Finally, our behavioral assay may have important applications in aquaculture research, since it could be easily implemented in other fish species with economic interest (e.g., sea bass, sea bream, salmon) by adapting the size of the behavioral apparatus.
Materials and Reagents
Adult zebrafish (Tübingen strain)
Acrylic dividers (Toyuto, catalog number: B07BF477K1)
Reverse osmosis purified water supplemented with 60 mg/L instant ocean sea salt (Aquariums Systems, catalog number: 210083)
Colored cards (white acrylic custom-made cards with green/red adhesive films)
Cultivated bloodworms (Ocean Nutrition, catalog number: I120E00)
Syringe 12 mL (Terumo, catalog number: SS-10S)
Airline tubing (Marina, catalog number: A1130)
Equipment
29×14×17 glass tanks (custom-made aquariums)
Half radial maze (custom-made aquarium, designed in-house; see Procedure for further details)
Camera for behavioral recording (HP, catalog number: HD 4310)
Two fish nets (Marina, catalog numbers: 11274, 11276; see Procedure for further details)
Software
Eyeline Video Surveillance System (NCH Software, version 2.06)
Observer XT (Noldus Technology, version 9)
Procedure
Fish housing through the procedure (Day 0)
General description: Fish (see Materials and Reagents #1) are kept in semi-isolation conditions (i.e., olfactory and visual cues from conspecifics are present) during the 24 h prior to the beginning of the behavioral protocol, to minimize any stress associated with individual testing (the zebrafish is a highly social species). Isolation tanks consist of 6 L tanks (Figure 1A, see Equipment #1) divided with transparent and perforated partitions (Figure 1B, see Materials and Reagents #2).
Fill isolation tanks with 5 L of system water (see Materials and Reagents #3) at 28°C, 750 μS, and pH 7.0. These water quality parameters are maintained through the procedure. System water of isolation tanks is renewed on a daily basis.
Select the experimental zebrafish that will be exposed to the judgment bias assay.
Place the selected fish in the individual compartments of the isolation tank (four fish per isolation tank). Fish are food-deprived in the isolation tanks.
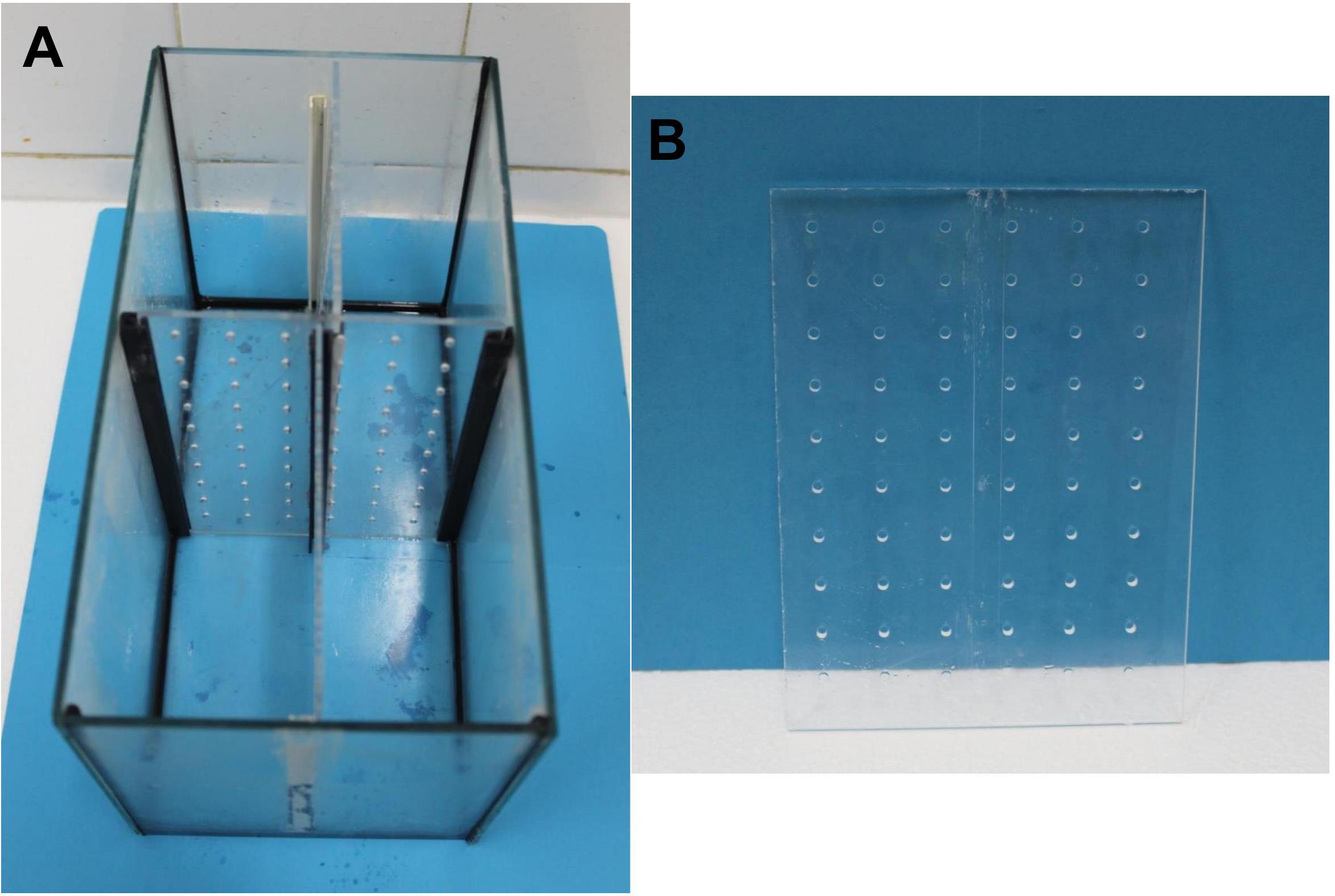
Figure 1. Material for fish housing.(A) Isolation tanks where fish are housed during the protocol. (B) Transparent, perforated dividers used in the isolation tanks in order to allow visual and olfactive cues from conspecifics.
Preparation of the behavioral apparatus (Day 1)
General description: The behavioral apparatus (see Equipment #2) consists of a half arm radial maze (Figure 2A) made of white acrylic. It contains removable colored cards (Figure 2B; see Materials and Reagents #4) placed at the end of each arm. Manually operated guillotine doors containing color cues (Figure 2C) separate the starting box from the arms of the radial maze. The two reference (training) arms [positive (P) and negative (N)] are positioned at 180° from each other and are equipped with full-colored cards (green or red). The three ambiguous (test) arms [near-positive (NP), ambiguous (A), and near-negative (NN)] are positioned at equidistant angles (each separated by 45°) between the two reference arms and contain mixed colored cards (green and red), with color proportions of 3:1, 1:1 and 1:3, respectively. The location of each arm is also considered as cue stimulus (in addition to color cues), since the central test arm (A) is located midway between P and N (90°), and the other two (NP and NN) halfway between A and each reference arm (45° and 135°, respectively). Researchers operate from behind a curtain, so that they are not visible to the experimental zebrafish during the behavioral assay. Visualization and recording of fish behavior is enabled by a personal computer linked to an overhead video camera (see Equipment#3). The judgment bias assay consists of three phases, which are performed over three consecutive days: habituation (day 1), training (two sessions, on days 1 and 2), and test (day 3) phases.
Fill the behavioral apparatus with 15 L of system water at 28°C, 750 μS, and pH 7.0.
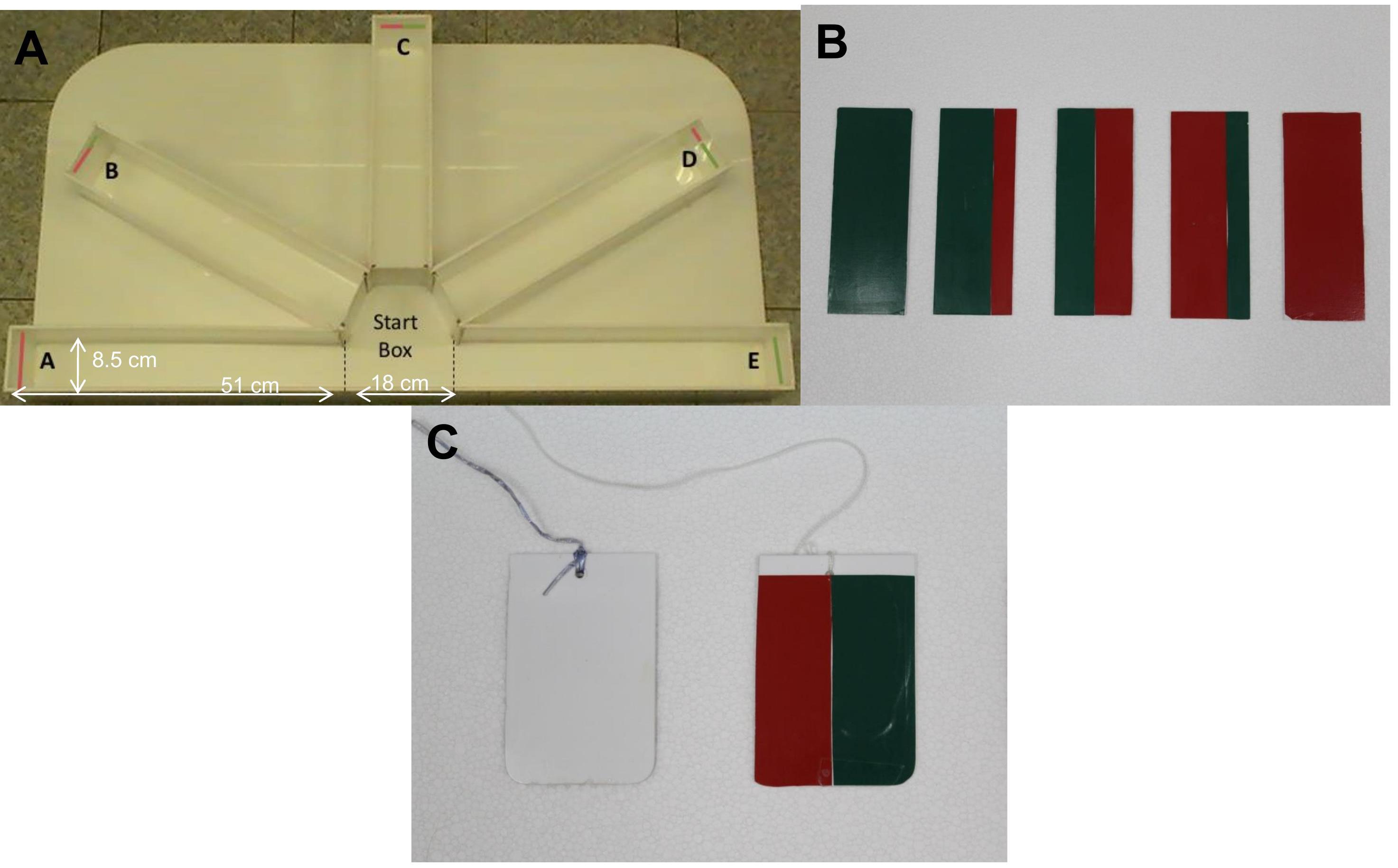
Figure 2. Behavioral apparatus.(A) Experimental setup showing the two reference locations [e.g., positive/rewarded (A) and negative/aversive (E)] and the three ambiguous locations [e.g., near-positive (B), ambiguous (C) and near-negative (D)]. (B) Removable colored cards are placed at the end of each arm. (C) Manually operated guillotine doors that link the starting box with each arm.
Habituation phase (day 1)
General description: Zebrafish are individually exposed to the behavioral apparatus for the first time during this phase. The time between the doors being opened and the fish leaving the arm (once the specific outcome is displayed) is recorded for each trial.
Transfer an individual from its isolation tank to the behavioral apparatus with the transportation net (see Equipment #4). All the doors of the behavioral setup are opened at the beginning of the session.
Note: It is important to have two different nets in size and color (Figure 3), to avoid transportation of the fish between tanks being wrongly associated with punishment by using the same net. A bigger black net is used for fish transport (transportation net), and a smaller green and white net is used for punishment (punishment net).

Figure 3. Nets used in the procedure of the judgment bias assay.Allow the fish to explore the whole tank for 10 min.
After 10 min, close all the doors and place the fish in the starting box of the experimental setup with the transportation net.
Randomly assign either left or right arm as the location for positive (P) training.
Randomly select either full green or full red as the color for positive (P) training. Add a fully colored card (green or red) to the previously selected arm.
Apply 1 min of inter-trial interval (ITI).
Manually open the guillotine door located at the entrance of the P arm to allow the fish to enter the arm.
Deliver a 1-mm piece of bloodworms (see Materials and Reagents #5) with the manual dispenser (Figure 4) at the end of the arm when the fish enters the arm. The manual dispenser consists of a 12 mL syringe (see Materials and Reagents #6) joined to a 3 mm transparent PVC tube (see Materials and Reagents #7). The dispenser allows the researchers to not be visible to the experimental zebrafish during this procedure.
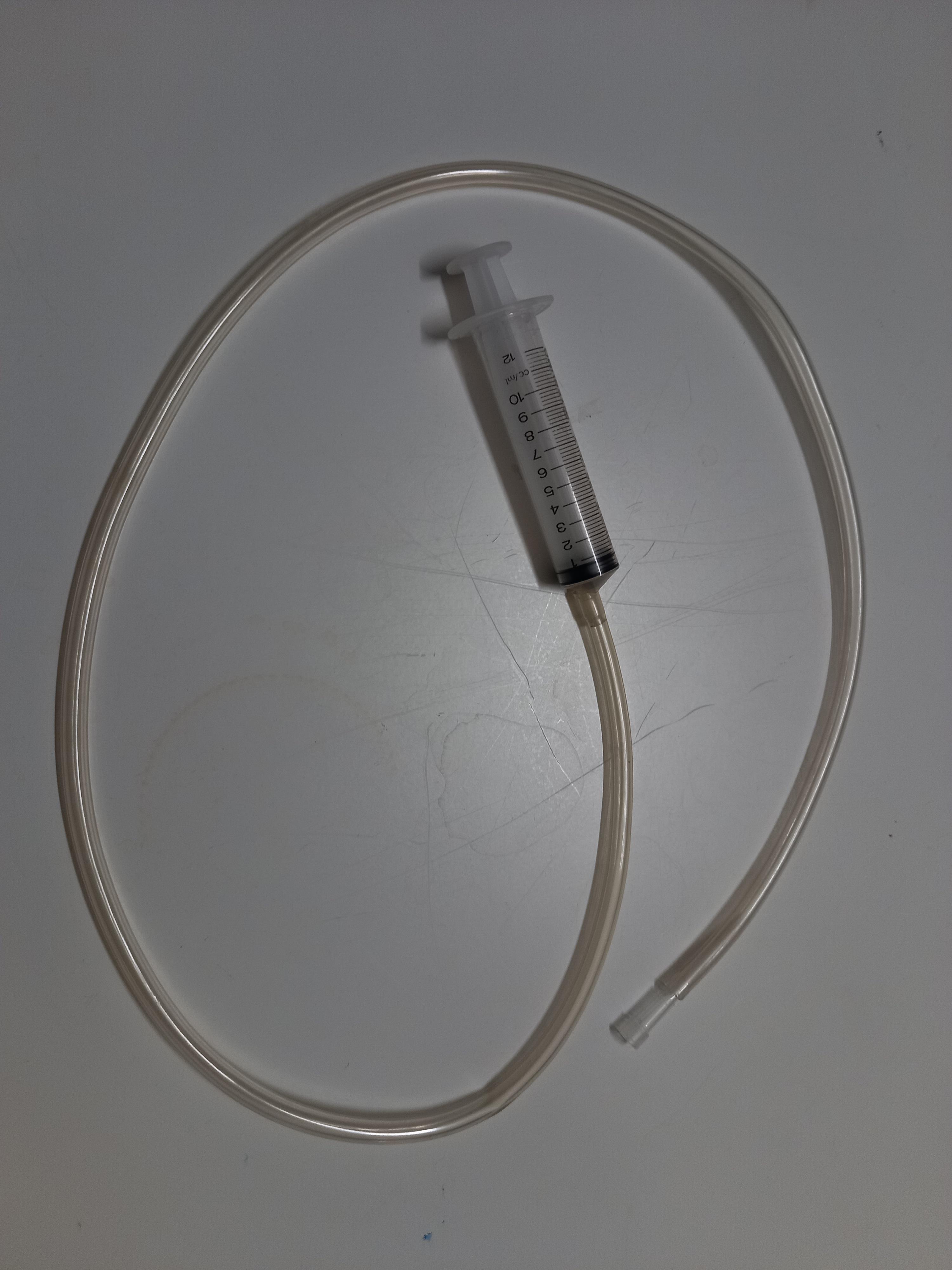
Figure 4. Manual dispenser used in the procedure of the judgment bias assay.Perform Steps C7–C8 for a total of four consecutive times, with 1 min of ITI between trials.
Return the fish to its isolation tank with the transportation net.
Change water between individuals.
Training phase session 1 (day 1)
General description: After habituation, fish are trained in a discrimination task for two consecutive days. The session 1 of the training phase is performed once the habituation phase ends for each experimental fish (inter-phase interval of 4–6 h). This phase consists of eight entries in total [four positive (P) and four negative (N)] in a pseudo-random sequence (i.e., P P N N P N P N). During this session, only one door is opened in each trial, either the one associated with the P (rewarded) or the N (aversive) outcome (Figure 5). The position (i.e., right or left) and the associated color cue (i.e., red or green) of the training arms (P and N) have to be counterbalanced between individuals. The time between the doors being opened and the fish leaving the arm (once the specific outcome is displayed) is recorded for each trial.
Transfer an individual from its isolation tank to the behavioral apparatus with the transportation net. All the doors of the behavioral setup are closed at the beginning of the session.
Place the fish in the starting box of the experimental setup for 1 min.
Manually open the guillotine door located at the entrance of the P arm to allow the fish to enter the arm.
Deliver a 1-mm piece of bloodworm with the manual dispenser at the end of the arm when the fish enter the arm.
Allow the fish to eat the piece of food and return to the starting box by itself. Close the guillotine door for the P arm and apply 1 min of ITI.
Manually open the guillotine door located at the entrance of the Negative (N) arm (including the second color cue; green or red) to allow the fish to enter the arm.
Perform a negative outcome (punishment) when the fish enters the N arm by catching the fish in the punishment net and gently shaking it for 10 s.
Allow the fish to return to the starting box and close the guillotine door of the N arm.
Perform Steps D3–D5 or D6–D8 for a total of eight consecutive times [four positive (P) and four negative (N)] in a pseudo-random sequence (i.e., P P N N P N P N).
Return the fish to its isolation tank with the transportation net.
Change water between individuals.
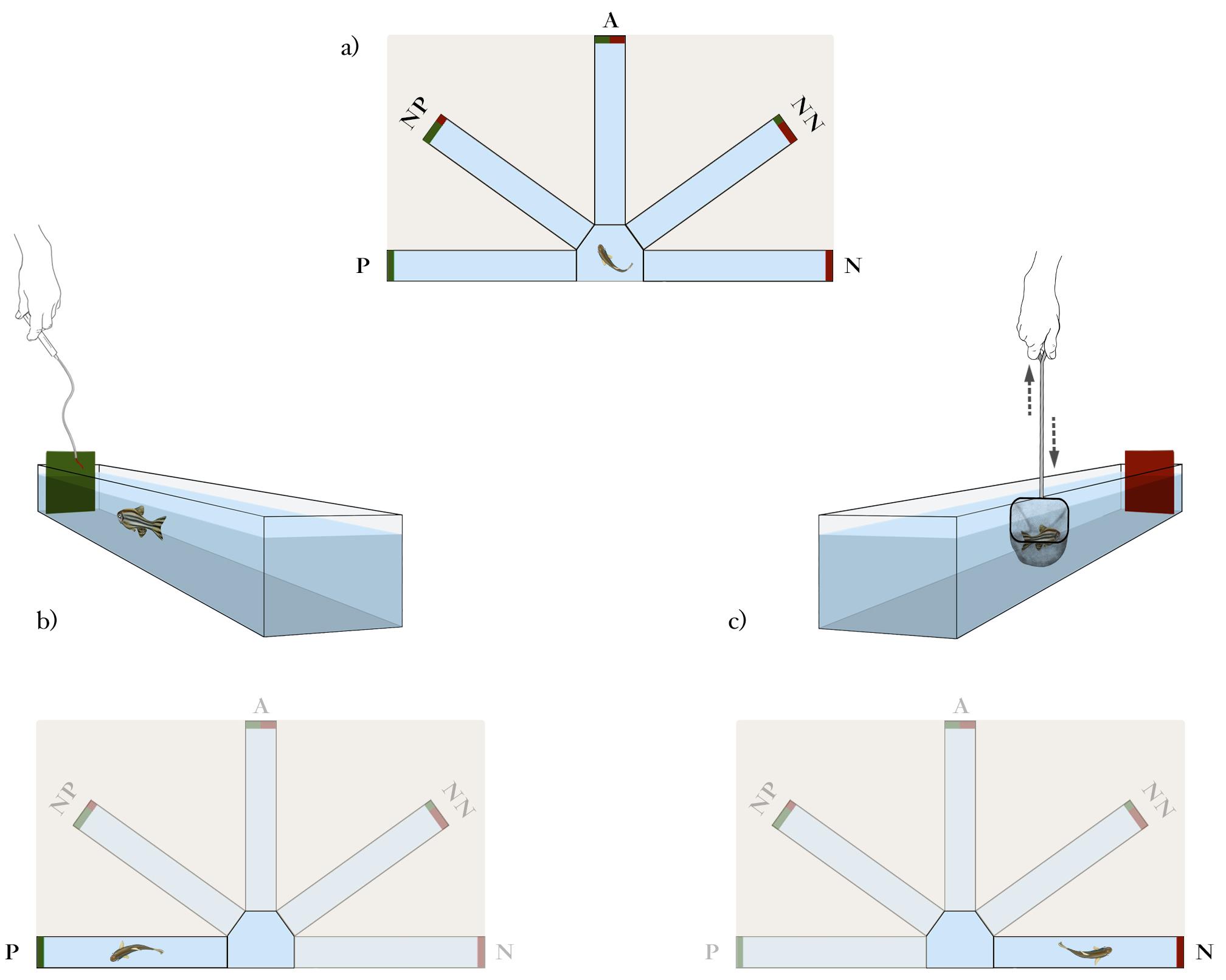
Figure 5. Schematic representation of the Training phase.(A) The fish is placed in the starting box of the experimental setup before starting each trial. (B) After 1 min of inter-trial interval (ITI), the guillotine door located at the entrance of the P arm is manually opened to allow the fish to enter the arm and receive the positive outcome (food reward; 1-mm piece of bloodworm), which is delivered at the end of the arm. Fish is then allowed to return by itself to the start box once the trial ends. (C) After an additional 1 min of ITI, the guillotine door located at the entrance of the N arm is manually opened to allow the fish to enter the arm and to receive the negavive outcome (punishment; fish is caught in a net inside the N arm and gently shaken in the net for 10 seconds). Eight entries [four positive (P) and four negative (N)] are performed in a pseudo-random sequence (i.e., P P N N P N P N).
Training phase session 2 (day 2)
General description: The second session of the training phase is performed on day 2 similarly to the first session.
Perform Steps D1–D11 of the training phase session 1 (see section D of Procedure).
Test phase (day 3)
General description: The test session consists of six pre-training trials (i.e., P P N N P N) followed by the presentation of cue tests. Each cue test is interspersed with one P and one N outcome presentation (i.e., training trials). Both a pre-training session and interspersed training trials were included in an attempt to ensure rapid and accurate discrimination of the task, by a potential enhancement of the short-term memory performance. In fact, the time that it took for the fish to learn the task was shortened, and the number of fish that learned the task was increased (personal observation from our pilot experiments). Therefore, the test sequence consisted of 19 trials in total (e.g., P P N N P N P P N NP N NP P N A P N NN; cue testing in bold). Reinforcements are absent in the cue tests. The order of presentation of the three ambiguous cues is counterbalanced between individuals. During this session, only one door is opened in each trial, either the one of the P (rewarded) or the N (aversive) outcome (Figure 6). The time between the doors being opened and the fish leaving the arm (once the specific outcome is displayed) is recorded for each trial.
Transfer an individual from its isolation tank to the behavioral apparatus with the transportation net. All the doors of the behavioral setup are closed at the beginning of the session.
Place the fish in the starting box of the experimental setup for 1 min.
Manually open the guillotine door located at the entrance of the P arm to allow the fish to enter the arm.
Deliver a 1-mm piece of bloodworm with the manual dispenser at the end of the arm, when the fish enter the arm.
Allow the fish to eat the piece of food and return to the starting box by itself. Close the guillotine door of the P arm and wait for 1 min of ITI.
Manually open the guillotine door located at the entrance of the Negative (N) arm (including the second color cue; green or red) to allow the fish to enter the arm.
Perform a negative outcome (punishment) when the fish enters the N arm, by catching the fish in the punishment net and gently shaking it for 10 s.
Allow the fish to return to the starting box and close the guillotine door of the N arm. Apply 1 min of ITI.
Perform Steps F3–F5 or F6–F8 for a total of six consecutive times [three positive (P) and three negative (N)] in a pseudo-random sequence (i.e., P P N N P N; pre-training trials). Apply 1 min of ITI between trials.
Manually open the guillotine door located at the entrance of the P arm to allow the fish to enter the arm (positive cue test). No reinforcement is delivered. Apply 1 min of ITI.
Perform Steps F3–F5 and F6–F8 [one positive (P) and one negative (N)] in a consecutive sequence (i.e., PN; interspersing training trials). Apply 1 min of ITI between trials.
Manually open the guillotine door located at the entrance of the N arm to allow the fish to enter the arm (Negative cue test). No reinforcement is delivered. Apply 1 min of ITI.
Perform Step F11.
Manually open manually the guillotine door located at the entrance of the NP arm to allow the fish to enter the arm (Near Positive cue test). No reinforcement is delivered. Apply 1 min of ITI.
Perform Step F11.
Manually open the guillotine door located at the entrance of the A arm to allow the fish to enter the arm (Ambiguous cue test). No reinforcement is delivered. Apply 1 min of ITI.
Perform Step F11.
Manually open the guillotine door located at the entrance of the NN arm to allow the fish to enter the arm (Near Negative cue test). No reinforcement is delivered. Apply 1 min of ITI.
Return the fish to its isolation tank with the transportation net.
Change water between individuals.
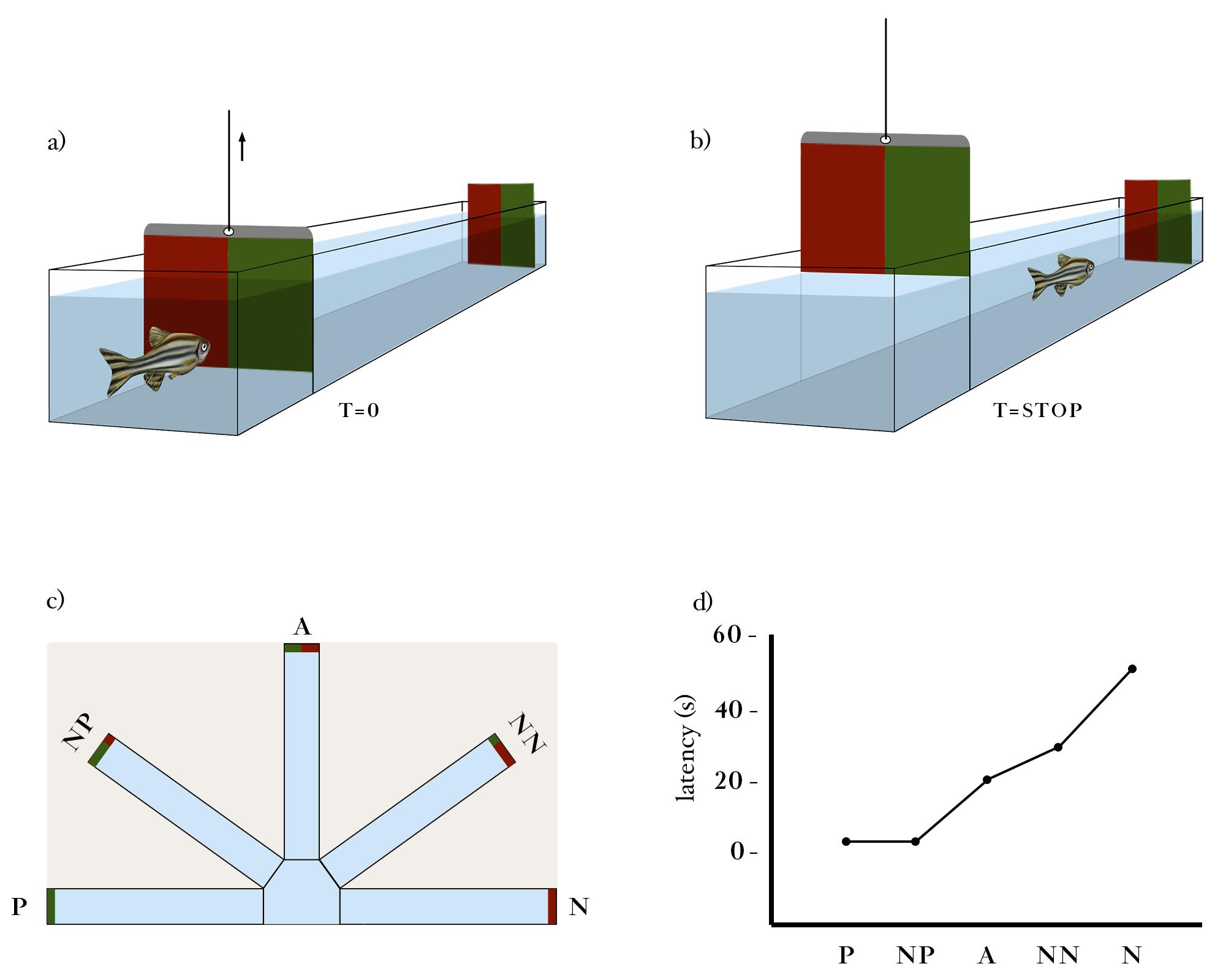
Figure 6. Schematic representation of the Test phase.The procedure for recording latencies consists of (A) starting the timing as the guillotine door is open and (B) stopping it as the fish enter the targeted arm. (C) The latency to enter in the target arm (60 s maximum) is recorded for each test trial (P, NP, A, NN, and N). The test sequence consists of 19 trials in total (e.g., P P N N P N P P N NP N NP P N A P N NN; test trials in bold). (D) Significant differences in the latency for each test trial are expected, with fish showing a lower latency to enter the P arm and progressively increasing it towards the color cue/location near N.
Data analysis
The latency to enter the target arm (60 s maximum) is recorded (see Software #1) and analyzed for each probe trial using a multi-event recorder software (see Software #2). Fish showing a difference <20 s in the latency between P and N (i.e., fail in the discrimination between P and N testing) are excluded from posterior analyses. In pilot experiments, we observed a consistent and well-differentiated response in the latencies between the learned stimuli tests (P and N), showing an average difference in latencies between P and N of 47.9 ± 6.1 s. We have established a threshold of 20 s to obtain a “window” wide enough to facilitate an accurate categorization of the ambiguous stimuli, since a narrow range could weaken the categorization of the individuals. In our previous study (Espigares et al., 2021), zebrafish showed significant differences in the latency to enter the different arms (P, NP, A, NN, and N), which reflected a generalization response, with fish showing a lower latency to enter P and progressively increasing the latency as the color cue/location neared N. If we assume that each full color (i.e., green or red) predicted a specific consequence (i.e., positive or negative; as it has been learned by the training with full colors), we can claim that the responses to the ambiguous cues are associated to color proportions instead of the recognition of just one color. Importantly, the A stimulus should not have any kind of bias (i.e., use always a fixed 1:1 color proportion) towards P or N, since this is the base on which fish are categorized in an optimistic/pessimistic dimension. For each individual fish, a judgment bias score (JBS) is calculated from the latencies (L) to enter the P, N, and A arms for each fish [JBS = (LA–LP)*100/(LN–LP)]. A JBS below 50 indicates an overall positive judgment and ‘optimistic’ interpretation of the ambiguous cue (fish perceived the ambiguous stimulus as a positive one), while higher JBS values indicate a negative (pessimistic) expectation bias (fish perceived the ambiguous stimulus as a negative one). However, selecting the extremes of the phenotype distribution (e.g., upper and lower quartiles of the JBS) would improve power to detect behavioral and/or physiological differences between phenotypes, since JBS in zebrafish falls into a bimodal distribution (unpublished data).
Notes
Due to the fundamental requirement for associative learning to successfully perform the discrimination task, stressed fish, as well as transgenic or mutant lines with impaired learning phenotypes, may not be suitable for performing the judgment bias assay. Furthermore, the amount of food delivered (i.e., 1-mm pieces of bloodworms) during each session was designed to avoid fish satiety. In fact, our previous results show that fish consumed all food rewards delivered throughout the assay. Fish not fully consuming food rewards at any point of the assay should be excluded from the experiment.
Acknowledgments
This work was supported by the BIAL Foundation through grant no. 130/12 awarded to R.F.O. and by a grant from Fundação para a Ciência e a Tecnologia (FCT, PTDC/BIA-COM/31010/2017 awarded to F.E. and R.F.O.). F.E. was supported by a Marie Skłodowska-Curie Actions - Individual Fellowship (H2020-MSCA-IF/703285) under the Horizon 2020 Framework Programme (H2020). The authors thank the Fish Facility Platform of the Instituto Gulbenkian de Ciência (Portugal) for animal care. The authors also acknowledge Lara Chapuis for the illustrations presented in Figures 5 and 6. This protocol was adapted from previous work (Espigares et al., 2021).
Competing interests
The authors declare they have no competing interests.
Ethics
All procedures were performed in accordance with Institutional and National regulations and guidelines, reviewed by the Ethics Committee of the Instituto Gulbenkian de Ciência, and approved by the competent Portuguese authority (Direcção Geral de Alimentação e Veterinária; permit no. 0421/000/000/2019).
References
- Baciadonna, L., and McElligott, A. G. (2015). The use of judgement bias to assess welfare in farm livestock. Anim Welf 24: 81-91.
- Bateson, M., Desire, S., Gartside, S. E. and Wright, G. A. (2011). Agitated honeybees exhibit pessimistic cognitive biases. Curr Biol 21(12): 1070-1073.
- Bethell, E. J. and Koyama, N. F. (2015). Happy hamsters? Enrichment induces positive judgement bias for mildly (but not truly) ambiguous cues to reward and punishment in Mesocricetus auratus. R Soc Open Sci 2(7): 140399.
- Boleij, H., van'tKlooster, J., Lavrijsen, M., Kirchhoff, S., Arndt, S. S. and Ohl, F. (2012). A test to identify judgement bias in mice. Behav Brain Res 233(1): 45-54.
- Carneiro, M. C., de Castro, I. P. and Ferreira, M. G. (2016). Telomeres in aging and disease: lessons from zebrafish. Dis Model Mech 9(7): 737-748.
- Douglas, C., Bateson, M., Walsh, C., Bédué, A., and Edwards, S. A. (2012). Environmental enrichment induces optimistic cognitive biases in pigs. Appl Anim Behav Sci 139(1-2): 65-73.
- Enkel, T., Gholizadeh, D., von Bohlen Und Halbach, O., Sanchis-Segura, C., Hurlemann, R., Spanagel, R., Gass, P. and Vollmayr, B. (2010). Ambiguous-cue interpretation is biased under stress- and depression-like states in rats. Neuropsychopharmacology 35(4): 1008-1015.
- Espigares, F., Abad-Tortosa, D., Varela, S. A. M., Ferreira, M. G. and Oliveira, R. F. (2021). Short telomeres drive pessimistic judgement bias in zebrafish. Biol Lett 17(3): 20200745.
- Harding, E. J., Paul, E. S. and Mendl, M. (2004). Animal behaviour: cognitive bias and affective state. Nature 427(6972): 312.
- Mendl, M., Brooks, J., Basse, C., Burman, O., Paul, E., Blackwell, E. and Casey, R. (2010). Dogs showing separation-related behaviour exhibit a 'pessimistic' cognitive bias. Curr Biol 20(19): R839-840.
- Murphy, E., Nordquist, R. E. and Josef, V. D. S., Franz (2013). Responses of conventional pigs and Göttingen miniature pigs in an active choice judgement bias task. Appl Anim Behav Sci 148(1-2): 64-76.
- Neave, H. W., Daros, R. R., Costa, J. H., von Keyserlingk, M. A. and Weary, D. M. (2013). Pain and pessimism: dairy calves exhibit negative judgement bias following hot-iron disbudding. PLoS One 8(12): e80556.
- Rygula, R., Szczech, E., Kregiel, J., Golebiowska, J., Kubik, J. and Popik, P. (2015). Cognitive judgment bias in the psychostimulant-induced model of mania in rats. Psychopharmacology (Berl) 232(3): 651-660.
- Salmeto, A. L., Hymel, K. A., Carpenter, E. C., Brilot, B. O., Bateson, M. and Sufka, K. J. (2011). Cognitive bias in the chick anxiety-depression model. Brain Res 1373: 124-130.
- Perry, C. J., Baciadonna, L. and Chittka, L. (2016). Unexpected rewards induce dopamine-dependent positive emotion-like state changes in bumblebees. Science 353(6307): 1529-1531.
- Stewart, A. M., Braubach, O., Spitsbergen, J., Gerlai, R. and Kalueff, A. V. (2014). Zebrafish models for translational neuroscience research: from tank to bedside. Trends Neurosci 37(5): 264-278.
- Tan, S. L. T. (2017). Cognitive bias as an indicator of emotional state and welfare in captive zebrafish. Dissertation, University of Melbourne.
- White, R., Rose, K. and Zon, L. (2013). Zebrafish cancer: the state of the art and the path forward. Nat Rev Cancer 13(9): 624-636.
Article Information
Publication history
Accepted: Dec 22, 2021
Published: Feb 20, 2022
Copyright
© 2022 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Espigares, F., Martins, R. R. and Oliveira, R. F. (2022). A Behavioural Assay to Investigate Judgment Bias in Zebrafish. Bio-protocol 12(4): e4327. DOI: 10.21769/BioProtoc.4327.
Category
Neuroscience > Behavioral neuroscience > Cognition
Biological Sciences
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Tips for asking effective questions
+ Description
Write a detailed description. Include all information that will help others answer your question including experimental processes, conditions, and relevant images.
Share
Bluesky
X
Copy link







