- Submit a Protocol
- Receive Our Alerts
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Skeletal Stem Cell Isolation from Cranial Suture Mesenchyme and Maintenance of Stemness in Culture
Published: Vol 12, Iss 5, Mar 5, 2022 DOI: 10.21769/BioProtoc.4339 Views: 1693
Reviewed by: Salma MerchantJunichi IwataValerie Uytterhoeven

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Human Schwann Cells in vitro I. Nerve Tissue Processing, Pre-degeneration, Isolation, and Culturing of Primary Cells
Gabriela I. Aparicio and Paula V. Monje
Nov 20, 2023 512 Views
Isolation, Purification, and Culture of Embryonic Melanoblasts from Green Fluorescent Protein–expressing Reporter Mice
Melissa Crawford [...] Lina Dagnino
Sep 5, 2023 331 Views
Generation of Mouse Primitive Endoderm Stem Cells
Yasuhide Ohinata [...] Haruhiko Koseki
Nov 20, 2023 238 Views
Abstract
Skeletal stem cells residing in the suture mesenchyme are responsible for calvarial development, homeostatic maintenance, and injury-induced repair. These naïve cells exhibit long-term self-renewal, clonal expansion, and multipotency. They possess osteogenic abilities to regenerate bones in a cell-autonomous manner and can directly replace the damaged skeleton. Therefore, the establishment of reliable isolation and culturing methods for skeletal stem cells capable of preserving their stemness promises to further explore their use in cell-based therapy. Our research team is the first to isolate and purify skeletal stem cells from the calvarial suture and demonstrate their potent ability to generate bone at a single-cell level. Here, we describe detailed protocols for suture stem cell (SuSC) isolation and stemness maintenance in culture. These methods are extremely valuable for advancing our knowledge base of skeletal stem cells in craniofacial development, congenital deformity, and tissue repair and regeneration.
Keywords: Bone regenerationBackground
Conventional methods have shown successful isolation of mesenchymal stromal cells (MSCs) from bone marrow and other tissues (Friedenstein et al., 1974; da Silva Meirelles et al., 2006). MSCs contain skeletal stem cells (SSCs) with self-renewing and skeletogenic differentiating abilities (Sacchetti et al., 2007). However, only ~10–20% of them are genuine SSCs (Robey et al., 2014). The difficulties in engraftment, survival, and differentiation of the transplanted MSCs have also been well documented (Caplan and Correa, 2011; Zeitouni et al., 2012). Furthermore, the cellular source of the endogenous MSC remains unknown. Here, we describe a protocol for the isolation of SSCs from the calvarial suture mesenchyme. These SuSCs have been demonstrated to generate bone at a single cell level upon kidney capsule transplantation, which can faithfully assess SuSC properties in vivo (Maruyama et al., 2016). It is also important to develop a protocol capable of maintaining their stemness in vitro. The successful establishment of the skeletal stem cell culture protocol permits further assessments of stem cell characteristics, e.g., self-renewal, proliferation, fate determination, and differentiation in an ex vivo setting. The preservation of stem cell stemness in culture is also critical for bone tissue engineering and opens the possibility of exploring next-generation therapeutics. We also describe an ex vivo protocol to culture SuSCs for an extended period. The cultured SuSCs can generate bones upon implantation to the ectopic site (Maruyama et al., 2021). We demonstrate that this culture method can determine the label-retaining ability and asymmetric division of SuSCs. It also provides an outstanding system to further our examination of additional stem cell characteristics, e.g., cell fate determination, generation of skeletal progenitors, and skeletogenic differentiation, as well as studies at the transcriptome (RNA-seq), epigenetics (ATAC-seq and ChIP-seq), and single-cell levels (scRNA-seq). Using these new tools, we can reconstruct lineage relationships between cells within craniofacial and skeletal tissues—a long-standing challenge in biology. These are extremely important advancements in stem cell-based therapy for bone regeneration and repair.
Materials and Reagents
Cell strainer 40 µm Nylon (Falcon, catalog number: 352340)
Ultra-Low Attachment Surface 24-well plate (Corning, catalog number: 3473)
Dulbecco’s Phosphate-Buffered Saline without calcium & magnesium (DPBS; Corning, catalog number: 21-031-CV), 2 years shelf life at room temperature
0.2% collagenase A (Roche, catalog number: 11088793001), freshly reconstituted from lyophilized stock in PBS
10 mM HEPES (1 M buffer solution; Gibco, catalog number: 15630-080), 24 months shelf-life at 4°C
Penicillin-Streptomycin (Gibco, catalog number: 15140-122), 12 months shelf life at -20°C
100 µg/mL transferrin (Sigma, catalog number: T8158) at -20°C
20 nM progesterone (Sigma, catalog number: P8783) at -20°C
30 nM Sodium selenite (Sigma, catalog number: S8295) at -20°C
60 nM Putrescine dihydrochloride (Sigma, catalog number: P5780), 4 years shelf life at -20°C
EGF (Mouse EGF Recombinant Protein; Gibco, catalog number: PMG8041), 1 year shelf life at -20°C
bFGF (mouse FGF-basic Recombinant Protein; Gibco, catalog number: PMG0035), 1 year shelf life at -20°C
B27 supplement (Gibco, catalog number: 17504044,), 1 year shelf life at -20°C
Insulin (Sigma, catalog number: I1882) at -20°C
0.25% trypsin (Gibco, catalog number: 25200-056), 2 years shelf life at -20°C
Soybean trypsin inhibitor (SBTI; Sigma, catalog number: T9128) at -20°C
Digestion Buffer (see Recipes)
Sphere Culture Media (see Recipes)
2× SBTI Solution (see Recipes)
Equipment
Shaker (Thermo Scientific, MaxQtm 4000 Benchtop Orbital Shakers)
Centrifuge (Thermo Scientific, Sorvall ST 16R Centrifuge)
Incubator (Thermo Forma, Series II Water Jacketed CO2 Incubator)
Procedure
Isolation of mesenchymal cells from suture mesenchyme
Dissect a 2 mm wide portion of calvarial tissue containing the sagittal (SAG) suture and its adjacent parietal bones from mouse calvarium (Figure 1).
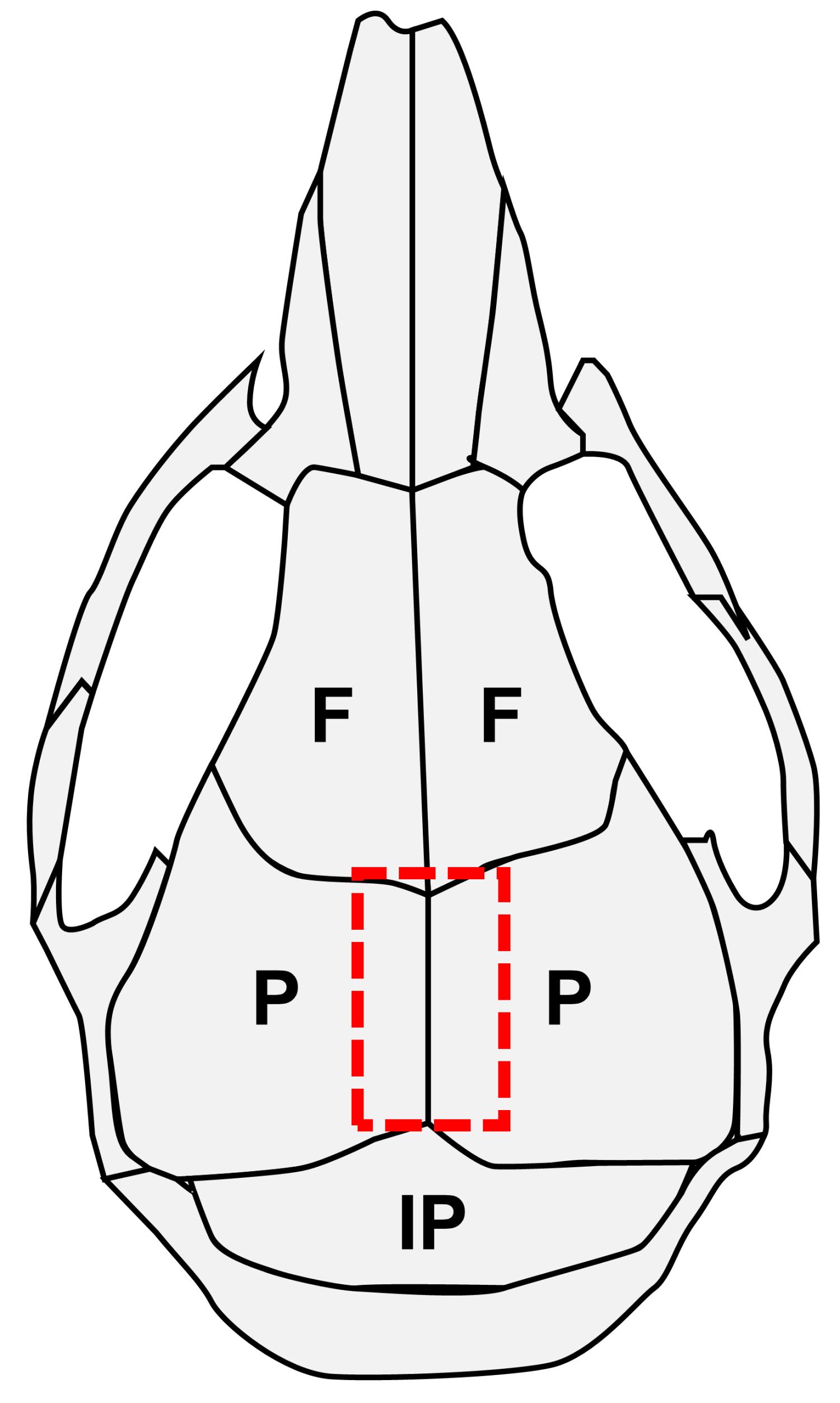
Figure 1. Schematic of mouse calvarial bones and sutures. F, frontal bone; P, parietal bone; IP, interparietal bone.Hint: It is easy to remove the calvarium from the meninges. The cutout size should be as small as possible, to limit the amounts of contaminant cells from the bone marrow and periosteum.
Grab both sides of the parietal bones with two forceps and gently pull them apart (Figure 2).
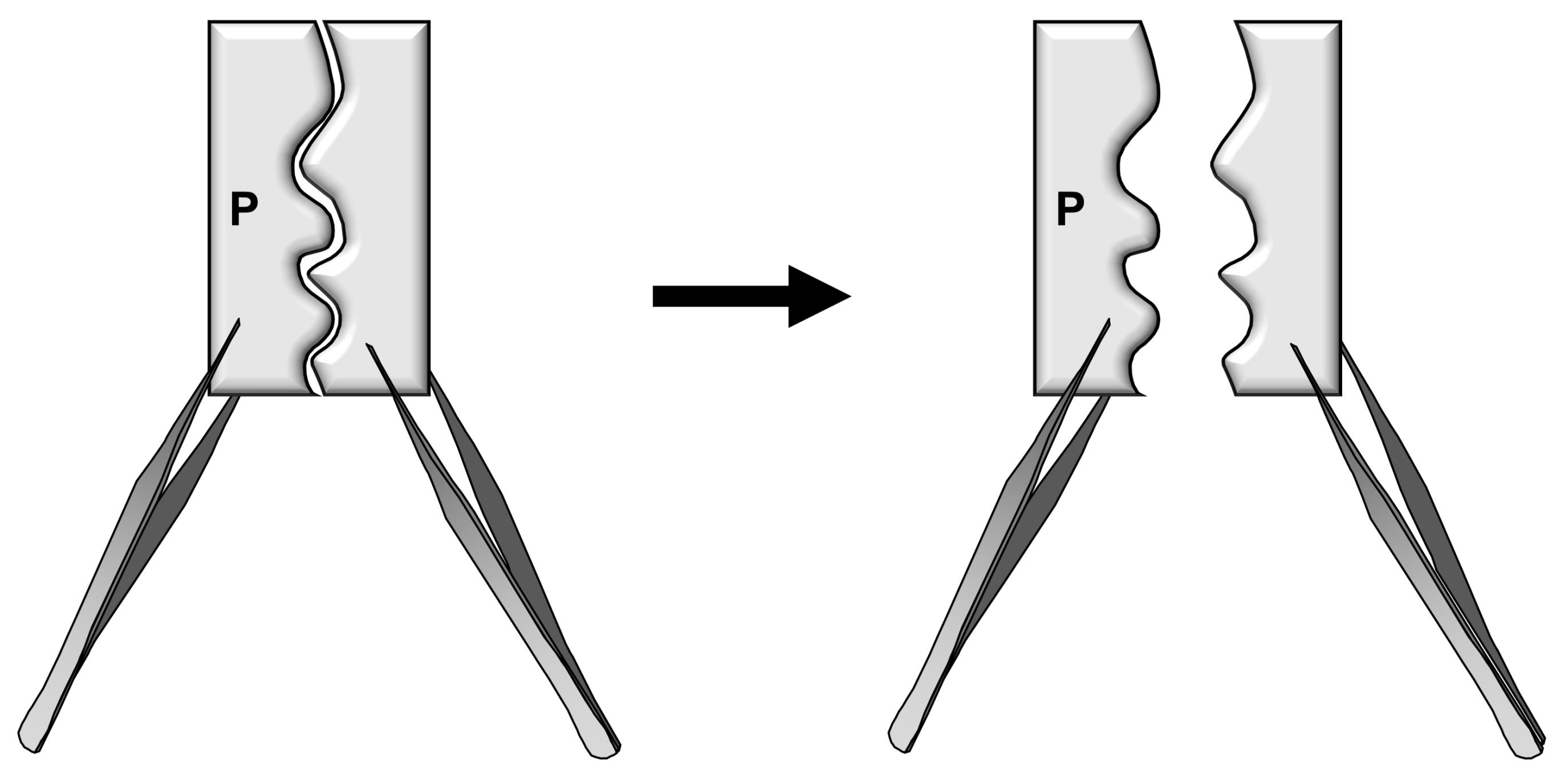
Figure 2. Schematic diagrams illustrating the separation of bone pieces to release the cells from the suture mesenchyme.Attention: The dissected calvarial tissue containing two small parietal bone pieces must be separated in the midline to expose the suture mesenchyme. Otherwise, the number of skeletal stem cells would be significantly reduced.
Hint: To avoid contamination of stem cells from the bone marrow, do not cut the separated calvarial tissue into pieces (this process is performed for calvarial cell isolation). Do not keep the dissected tissue on ice, rather quickly incubate it with Digestion Buffer as described in step 6.
Submerge the separated pieces in 40 mL of Digestion Buffer and incubate in a shaker at 200 × g (MaxQtm 4000 Benchtop Orbital Shakers) and 37°C for 1 h.
Filter the dissociated cells through a 40 µm strainer.
Spindown the cells by centrifugation at 400 × g and 4°C for 7 min.
Remove the supernatant to collect the cell pellets.
Hint: About 5 × 104 cells can be obtained from one sagittal suture
Hint: Suture mesenchymal cells have been successfully isolated from the calvaria of mice at postnatal day 10 (P10) and P28.
Sphere culture
Resuspend the cell pellets with Sphere Culture Media and count the cell number.
Seed the cells in 2 mL of sphere culture media at 104–105 cells per well on an Ultra-Low Attachment Surface 24-well plate (Figure 3A).
Carefully change half the amount of the culture media in the culture every other day, without disturbance of cell settling.
Hint: Sphere formation will be visible within 10 to 14 days (Figure 3B and Maruyama et al., 2021).
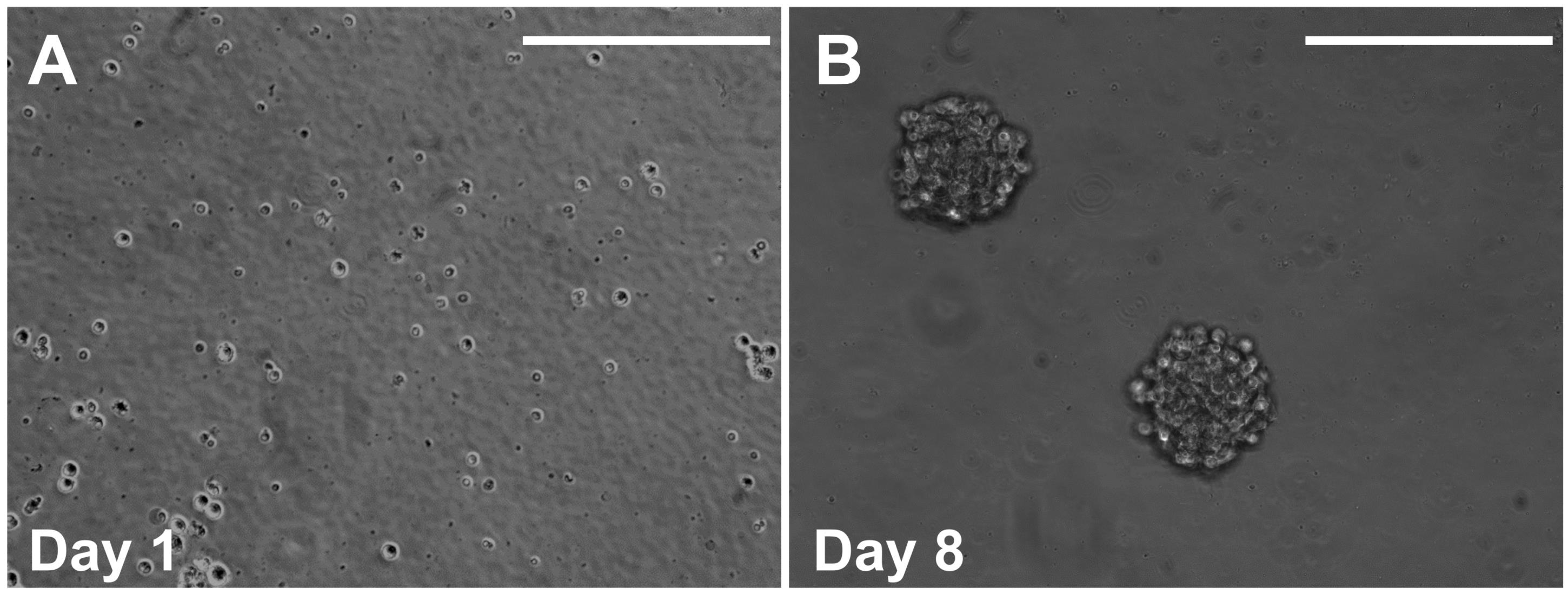
Figure 3. Representative images showing cells after 1 day (A) and 8 days (B) of the sphere culture. Scale bars, 200 µm.Hint: Most of the sphere is derived from a single stem cell (Maruyama et al., 2021).
Hint: Stem cell stemness, e.g., bone-forming ability and quiescence, has been demonstrated to be maintained in culture using the 1st and 3rd passage of the sphere by kidney capsule transplantation and label-retaining analyses (Maruyama et al., 2021).
Hint: Bone-forming ability is assessed by the kidney capsule transplantation assay, followed by von Kossa staining and immunostaining of osteoblast markers. Quiescence is examined by the pulse-chase assay in sphere culture.
Passaging spheres
Some spheres may be weakly attached to the bottom of the well. To detach the sphere, gently pipette the culture media up and down.
The cell suspension is collected by centrifugation at 300 × g and 4°C for 7 min.
Remove the supernatant and tap the tube to gently break up the pellets.
Add 1 mL of 0.25% Trypsin to resuspend the pellet, followed by incubation at 37°C for 5 min.
Add 1 mL of 2× SBTI solution to stop the activity of the trypsin.
Centrifuge at 300 × g and 4°C for 7 min.
Remove supernatant and tap the tube to gently break up the pellets.
Resuspend the cells in the Sphere Culture Media and count cell numbers.
Seed the cells at 104–105 cells per well.
Hint: This culture method has been limited to five passages.
Data analysis
Sphere measurement
Gently pipette the medium to detach the spheres from the culture plate.
Hint: No special pipet tips are required, only general ones that generate a little media flow.
Take images using a camera under a microscope, e.g., NIKON CB-115 (Figures 5, 6, Supplemental Figures S9, S10 of Maruyama et al., 2021).
Measure the sphere number and size by ImageJ (Figures 6, Supplemental Figures S9, S10 of Maruyama et al., 2021).
Hint: For the basic function of ImageJ: “ImageJ Basics (PDF file)” on the official page (https://imagej.nih.gov/ij/index.html) under “Documentation”-“Tutorials and Examples”.
Count spheres that are >20 µm in diameter.
Perform at least three independent experiments for statistical evaluation.
Examine the statistical significance of sphere number and size using a two-sided Student’s t-test.
Evaluation of bone formation after kidney capsule transplantation
Perform whole-mount von Kossa staining of bone in the transplanted kidney capsule (Figures 4, 5, 6, Supplemental Figures S9, S10 of Maruyama et al., 2021).
Image using a microscope, e.g., NIKON CB-115.
Measure the size of the stained area by ImageJ.
Perform at least three independent experiments for statistical evaluation.
Examine the statistical significance of sphere number and size using a two-sided Student’s t-test (Supplemental Figure S10 of Maruyama et al., 2021).
Recipes
Digestion Buffer (40 mL)
DPBS containing 0.2% collagenase A, 1% Penicillin-Streptomycin, and 10 mM HEPES
Sphere Culture Media
25 µg/mL insulin
100 µg/mL transferrin
20 nM progesterone
30 nM Sodium selenite
60 nM putrescine
20 ng/mL EGF
20 ng/ml bFGF
1× B27 supplement
1% Penicillin-Streptomycin
2× SBTI Solution
2 mg SBTI in 1 mL of DPBS
Acknowledgments
The authors thank former and current lab members for their technical and intellectual support. This work is supported by the National Institutes of Health (DE15654, DE269369) and NYSTEM (C029558) to W.H. This protocol is derived from the original research paper published in Science Translational Medicine (Maruyama et al., 2021).
Competing interests
The authors declare no competing financial interests.
Ethics
Care and use of experimental animals described in this work comply with guidelines and policies of the University Committee on Animal Resources at the University of Rochester and IACUC at the Forsyth Institute.
References
- Caplan, A. I. and Correa, D. (2011). The MSC: an injury drugstore. Cell Stem Cell 9(1): 11-15.
- da Silva Meirelles, L., Chagastelles, P. C. and Nardi, N. B. (2006). Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci (11): 2204-2213.
- Friedenstein, A. J., Chailakhyan, R. K., Latsinik, N. V., Panasyuk, A. F. and Keiliss-Borok, I. V. (1974). Stromal cells responsible for transferring the microenvironment of the hemopoietic tissues. Cloning in vitro and retransplantation in vivo. Transplantation 17(4): 331-340.
- Maruyama, T., Jeong, J., Sheu, T. J. and Hsu, W. (2016). Stem cells of the suture mesenchyme in craniofacial bone development, repair and regeneration. Nat Commun 7: 10526.
- Maruyama, T., Stevens, R., Boka, A., DiRienzo, L., Chang, C., Yu, H. I., Nishimori, K., Morrison, C. and Hsu, W. (2021). BMPR1A maintains skeletal stem cell properties in craniofacial development and craniosynostosis. Sci Transl Med 13(583): eabb4416.
- Robey, P. G., Kuznetsov, S. A., Riminucci, M. and Bianco, P. (2014). Bone marrow stromal cell assays: in vitro and in vivo. Methods Mol Biol 1130: 279-293.
- Sacchetti, B., Funari, A., Michienzi, S., Di Cesare, S., Piersanti, S., Saggio, I., Tagliafico, E., Ferrari, S., Robey, P. G. and Riminucci, M. (2007). Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell 131(2): 324-336.
- Zeitouni, S., Krause, U., Clough, B. H., Halderman, H., Falster, A., Blalock, D. T., Chaput, C. D., Sampson, H. W. and Gregory, C. A. (2012). Human mesenchymal stem cell-derived matrices for enhanced osteoregeneration. Sci Transl Med 4(132): 132ra155.
Article Information
Publication history
Accepted: Jan 9, 2022
Published: Mar 5, 2022
Copyright
© 2022 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Maruyama, T., Yu, H. I. and Hsu, W. (2022). Skeletal Stem Cell Isolation from Cranial Suture Mesenchyme and Maintenance of Stemness in Culture. Bio-protocol 12(5): e4339. DOI: 10.21769/BioProtoc.4339.
- Maruyama, T., Stevens, R., Boka, A., DiRienzo, L., Chang, C., Yu, H. I., Nishimori, K., Morrison, C. and Hsu, W. (2021). BMPR1A maintains skeletal stem cell properties in craniofacial development and craniosynostosis. Sci Transl Med 13(583): eabb4416.
Category
Stem Cell > Adult stem cell
Cell Biology > Cell isolation and culture > Cell isolation
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Tips for asking effective questions
+ Description
Write a detailed description. Include all information that will help others answer your question including experimental processes, conditions, and relevant images.
Share
Bluesky
X
Copy link