- Submit a Protocol
- Receive Our Alerts
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
In vitro Assays to Evaluate Specificity and Affinity in Protein-phospholipid Interactions
Published: Vol 12, Iss 10, May 20, 2022 DOI: 10.21769/BioProtoc.4421 Views: 1648
Reviewed by: Laxmi Narayan MishraNityanand SrivastavaSashi ReddyAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols
A Chemiluminescence Based Receptor-ligand Binding Assay Using Peptide Ligands with an Acridinium Ester Label
Mari Wildhagen [...] Markus Albert
Mar 20, 2015 10565 Views
Protein Immunoprecipitation Using Nicotiana benthamiana Transient Expression System
Fang Xu [...] Xin Li
Jul 5, 2015 20962 Views
Mating Based Split-ubiquitin Assay for Detection of Protein Interactions
Wijitra Horaruang and Ben Zhang
May 5, 2017 14741 Views
Abstract
Protein–lipid interactions play important roles in many biological processes, including metabolism, signaling, and transport; however, computational and structural analyses often fail to predict such interactions, and determining which lipids participate in these interactions remains challenging. In vitro assays to assess the physical interaction between a protein of interest and a panel of phospholipids provide crucial information for predicting the functionality of these interactions in vivo. In this protocol, which we developed in the context of evaluating protein–lipid binding of the Arabidopsis thaliana florigen FLOWERING LOCUS T, we describe four independent in vitro experiments to determine the interaction of a protein with phospholipids: lipid–protein overlay assays, liposome binding assays, biotin-phospholipid pull-down assays, and fluorescence polarization assays. These complementary assays allow the researcher to test whether the protein of interest interacts with lipids in the test panel, identify the relevant lipids, and assess the strength of the interaction.
Keywords: Protein-phospholipids interactionBackground
Protein–lipid interactions form the basis of many cellular functions. In particular, interactions involving lipids in the plasma membrane and other cellular membranes have essential functions in transport, signaling, and metabolism. Proteins also interact with lipids in lipid biosynthesis; moreover, lipid catabolism provides energy for animals and for seed germination in many plants. Despite the importance of protein–lipid interactions, many such interactions are not predicted from protein sequences and structural analysis (Yao et al., 2013; Kim et al., 2019). Moreover, identifying the specific lipid(s) involved in these interactions remains challenging.
In addition to their well-known roles in other processes, protein–phospholipid interactions modulate flowering time in Arabidopsis thaliana (Nakamura et al., 2014; Susila et al., 2021). In this process, FLOWERING LOCUS T (FT) protein specifically interacts with phosphatidylglycerol (PG) and is sequestered into the membrane to prevent precocious flowering, especially at low ambient temperature (Susila et al., 2021). FT belongs to the phosphatidylethanolamine binding protein (PEBP) family and has a conserved anion-binding pocket that could be responsible for binding PG (Bernier et al., 1986; Jin et al., 2021). Lipid binding also affects other regulatory factors; for example, several transcription factors without transmembrane domains interact with phosphatidic acid (PA) to regulate plant development and stress responses (Yao et al., 2013; Kim et al., 2019). Research on phospholipid-binding proteins may uncover additional novel regulatory mechanisms in complex biological systems.
Here, we describe four methods that we used to screen for interactions of FT with phospholipids: 1) lipid–protein overlay assays, 2) liposome binding assays, 3) biotin-phospholipid pull-down assays, and 4) fluorescence polarization assays (Susila et al., 2021). We believe that using different in vitro methods is necessary to validate the protein–phospholipid interactions. This versatile protocol can be used for any protein of interest, although the incubation temperature and the duration of incubation should be tested for each protein. These assays provide complementary information on protein–lipid interactions, including whether the protein of interest interacts with the lipids in the test panel, which lipid(s) it prefers, and how strongly it interacts with each species of lipid.
Materials and Reagents
Materials
Recombinant FT protein was purified from Escherichia coli following standard methods (Susila et al., 2021). The phospholipids and galactolipids were obtained from Sigma-Aldrich or Avanti Polar Lipids (Table 1).
Table 1. Lipids used in this study
Lipid Abbreviation Vendor Catalog number 1,2-dipalmitoyl-sn-glycero-3-phosphate DPPA Avanti Polar Lipids 830855P 1,2-dipalmitoyl-sn-glycero-3-phospho-1'-myo-inositol) DPPI Avanti Polar Lipids 850141P 1,2-dipalmitoyl-sn-glycero-3-phospho-1'-rac-glycerol) DPPG Avanti Polar Lipids 840455P 1,2-dipalmitoyl-sn-glycero-3-phospho-L-serine DPPS Avanti Polar Lipids 840037P 1,2-dipalmitoyl-sn-glycero-3-phosphocholine DPPC Avanti Polar Lipids 850355 1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine DPPE Avanti Polar Lipids 850705 L-α-phosphatidyl-DL-glycerol ammonium salt from egg yolk lecithin PG Sigma-Aldrich P0514 L-α-phosphatidylethanolamine from egg yolk PE Sigma-Aldrich P7943 1-oleoyl-2-[12-biotinyl(aminododecanoyl)]-sn-glycero-3-phospho-(1'-rac-glycerol) (sodium salt) tBiotin-PG Avanti Polar Lipids 860581C 1-oleoyl-2-[12-biotinyl(aminododecanoyl)]-sn-glycero-3-phosphocholine tBiotin-PC Avanti Polar Lipids 860563C Lipid–protein overlay assay
Volac Pasteur pipette (Sigma-Aldrich, catalog number: Z310727)
Polyvinylidene fluoride (PVDF) membrane (Millipore, catalog number: IPVH304F0)
X-ray film (AGFA, catalog number: CP-BU New)
Chloroform (Merck, catalog number: 1.02445.1000)
Fatty acid-free bovine serum albumin (Sigma-Aldrich, catalog number: A6003)
AntiHis monoclonal antibody (Sigma-Aldrich, catalog number: H1029; 1:2,000 dilution)
Anti-Strep-tag II monoclonal antibody (Abcam, catalog number: ab76949; 1:2,000 dilution)
Goat anti-mouse IgG (Thermo Fisher Scientific, catalog number: A28177; 1:10,000 dilution)
Western Blotting Detection Reagent Kit Ab Signal (AbClon, catalog number: Abc-3001)
Tris (Duchefa, catalog number: T1501)
NaCl (Duchefa, catalog number: S0520)
Glycerol (Duchefa, catalog number: G1345)
2-mercaptoethanol (Sigma-Aldrich, catalog number: M6250)
Purified tagged protein (His-FT or FT-StII in dialysis buffer; see Recipes)
Tris-buffered saline (TBS) (see Recipes)
Liposome binding assay
Volac Pasteur pipette (Sigma-Aldrich, catalog number: Z310727)
Protein LoBind Tubes (Eppendorf, catalog number: 0030108116)
PVDF membrane (Millipore, catalog number: IPVH304F0)
X-ray film (AGFA, catalog number: CP-BU New)
Chloroform (Merck, catalog number: 1.02445.1000)
Skim milk (Sigma-Aldrich, catalog number: 70166)
Bovine serum albumin (MP Biomedicals, catalog number: 0216006980)
AntiHis monoclonal antibody (Sigma-Aldrich, catalog number: H1029; 1:2,000 dilution)
Goat anti-mouse IgG (Thermo Fisher Scientific, catalog number: A28177; 1:10,000 dilution)
Western blotting Detection Reagent Kit Ab Signal (AbClon, catalog number: Abc-3001)
SDS (Sigma-Aldrich, catalog number: L5750)
Bromophenol blue (Sigma-Aldrich, catalog number: B0126)
Purified tagged protein (His-FT or FT-StII in dialysis buffer; see Recipes)
Tris-buffered saline (TBS) (see Recipes)
SDS sample buffer (see Recipes)
Biotin-phospholipid pull-down assay
Volac Pasteur pipette (Sigma-Aldrich, catalog number: Z310727)
Protein LoBind Tubes (Eppendorf, catalog number: 0030108116)
PVDF membrane (Millipore, catalog number: IPVH304F0)
X-ray film (AGFA, catalog number: CP-BU New)
Chloroform (Merck, catalog number: 1.02445.1000)
Ethanol (Merck, catalog number: 1.00983.1011)
Dynabeads MyOne Streptavidin T1 (Invitrogen, catalog number: 65601)
Skim milk (Sigma-Aldrich, catalog number: 70166)
Bovine serum albumin (MP Biomedicals, catalog number: 0216006980)
AntiHis monoclonal antibody (Sigma-Aldrich, catalog number: H1029; 1:2,000 dilution)
Goat anti-mouse IgG (Thermo Fisher Scientific, catalog number: A28177; 1:10,000 dilution)
Western blotting Detection Reagent Kit Ab Signal (AbClon, catalog number: Abc-3001)
SDS sample buffer (see Recipes)
Purified tagged protein (His-FT in dialysis buffer; see Recipes)
Tris-buffered saline (TBS) (see Recipes)
Fluorescence polarization assay
Volac Pasteur pipette (Sigma-Aldrich, catalog number: Z310727)
Protein LoBind Tubes (Eppendorf, catalog number: 0030108116)
96-well black flat-bottom polystyrene plate (Corning, catalog number: 3915)
PierceTM NHS-Rhodamine Antibody Labeling Kit (ThermoFisher Scientific, catalog number: 53031)
Chloroform (Merck, catalog number: 1.02445.1000)
KCl (Sigma Aldrich, catalog number: P3911)
Na2HPO4 (Sigma-Aldrich, catalog number: S9763)
KH2PO4 (Sigma-Aldrich, catalog number: P0662
Purified tagged protein (His-FT in PBS dialysis buffer; see Recipes)
Phosphate-buffered saline (PBS) (see Recipes)
PBS elution buffer (see Recipes)
Equipment
Lipid–protein overlay assay
Amber glass vial (Shimadzu Scientific Korea, catalog number: 91005-0920)
Vortex mixer (Scientific Industries, Genie 2, model: G-560)
Micropipette (Eppendorf Research, catalog number: 3123000918)
Western blot tray
Orbital shaker (Best of Lab Equipments, model: RF300)
X-ray film cassette
X-ray film processor (Healthcare Solutions Inc., model: JP-33)
Liposome binding assay
Amber glass vial (Shimadzu Scientific Korea, catalog number: 91005-0920)
Vortex mixer (Scientific Industries, Genie 2, model: G-560)
Micropipette (Eppendorf Research, catalog number: 3123000918)
Bioruptor sonication device (Bio-Medical Science, model: KRB 01)
Refrigerated centrifuge (Eppendorf Research, model: 5430R)
Incubator (Vision Scientific, model: VS-8480MX2-DT)
Heat block (Best of Lab Equipments, model: CF1)
Western blot tray
Orbital shaker (Best of Lab Equipments, model: RF300)
X-ray film cassette
X-ray film processor (Healthcare Solutions Inc., model: JP-33)
Biotin-phospholipid pull-down assay
Amber glass vial (Shimadzu Scientific Korea, catalog number: 91005-0920)
Vortex mixer (Scientific Industries, Genie 2, model: G-560)
Micropipette (Eppendorf Research, catalog number: 3123000918)
Tube rotator (Seoulin Bioscience, model: SLRM-3)
Magnetic separation rack
Spin-down centrifuge (Allsheng, model: mini-6K)
Heat block (Best of Lab Equipments, model: CF1)
Western blot tray
Orbital shaker (Best of Lab Equipments, model: RF300)
X-ray film cassette
X-ray film processor (Healthcare Solutions Inc., model: JP-33)
Fluorescence polarization assay
Amber glass vial (Shimadzu Scientific Korea, catalog number: 91005-0920)
Vortex mixer (Scientific Industries, Genie 2, model: G-560)
Micropipette (Eppendorf Research, catalog number: 3123000918)
Bioruptor Sonication device (Bio-Medical Science, model: KRB 01)
Refrigerated centrifuge (Eppendorf Research, model: 5430R)
Incubator (Vision Scientific, model: VS-8480MX2-DT)
SpectraMax Multi-Mode Plate Reader (Molecular Devices)
Software
ImageJ Fiji, an open source software (Schindelin et al., 2012)
GraphPad Prism 5 (Graph Pad Software Inc.)
Procedure
Lipid–protein overlay assay
In this qualitative assay, lipids are immobilized on a membrane and incubated with tagged protein; any bound protein is then detected by antibodies to the epitope tag (Figure 1 and Figure 5A). We recommend the use of a tag protein (e.g., GST, GFP, or MBP) or a protein without lipid-binding capacity as a negative control for the experiment.
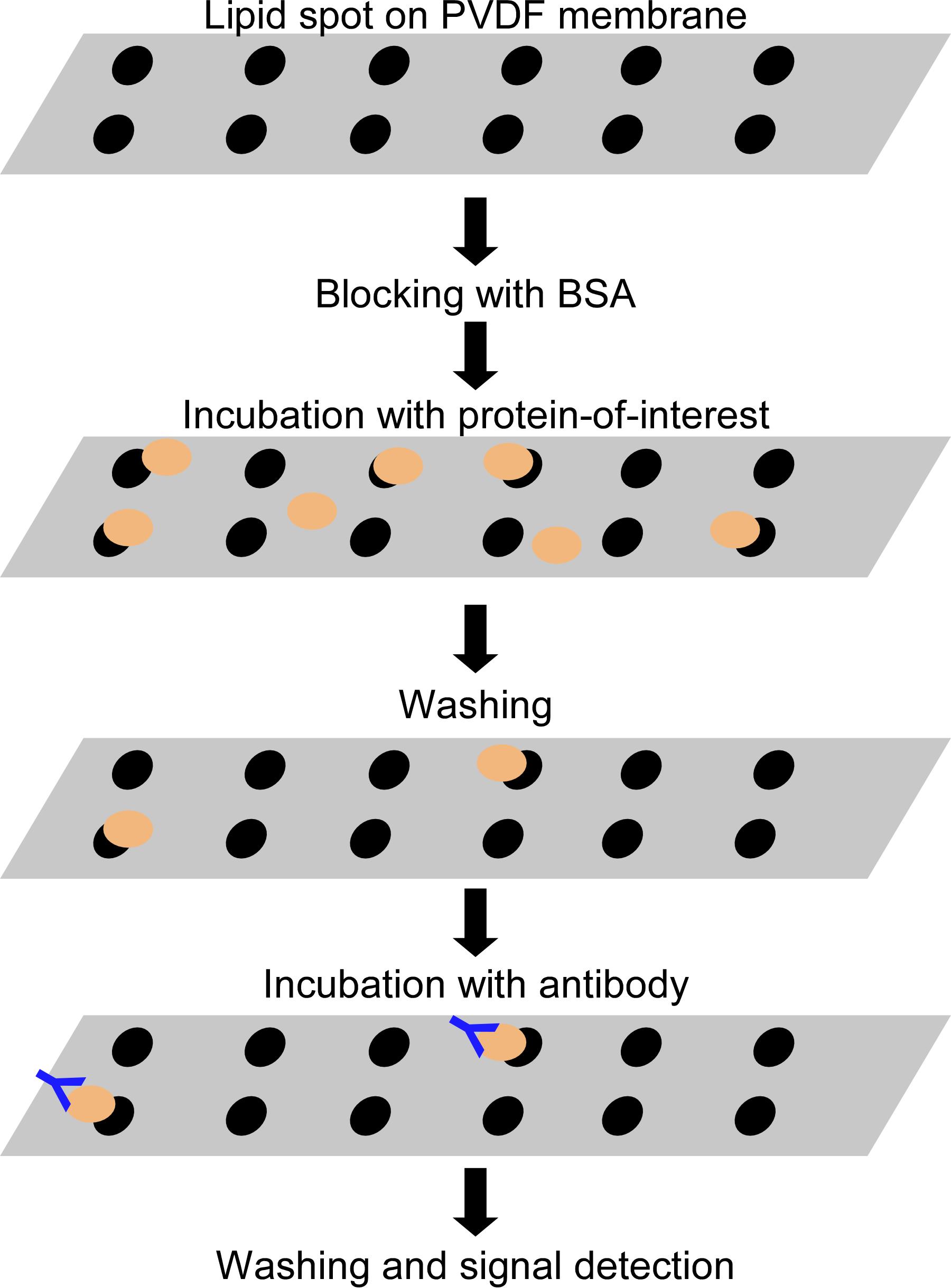
Figure 1. Lipid–protein overlay assay. Lipids are immobilized on a PVDF membrane and incubated with the protein of interest. Lipid-bound proteins are detected with western blotting.Dissolve the lipids in pure chloroform to prepare stock solutions (1 mg/mL). Use a Volac Pasteur pipette to mix and transfer the lipid solutions.
Store the lipid stock solutions in amber glass vials at -20°C.
Cut the PVDF membrane (2 × 8 cm) and mark the spots (at least 1 cm apart) for the lipids.
Slowly spot 5 µg of each lipid (5 µL of stock solution) onto the prepared PVDF membrane (at least 1–2-mm diameter) and dry the membrane for at least 30 min to 1 h at room temperature.
Place the membrane in the western blot tray (6 × 10 cm) and incubate with 10 mL of blocking solution (TBS containing 3% [w/v] fatty acid-free bovine serum albumin), at room temperature for 1 h with gentle agitation (10 rpm).
Discard the blocking solution and incubate the membrane with 60 µg of recombinant His-FT or FT-StII protein in 5 mL of blocking solution at 4°C for 16 h with gentle agitation (10 rpm).
Discard the protein solution and wash the membrane with 10 mL of TBS for 5 min; repeat the washes a total of four times.
Incubate the membrane with 10 mL blocking solution containing the primary antibody, either antiHis monoclonal antibody or Anti-Strep-tag II monoclonal antibody, at room temperature for 1 h with gentle agitation (10 rpm).
Discard the antibody solution and wash the membrane with 10 mL of TBS for 5 min; repeat the washes four times.
Incubate the membrane with 10 mL of blocking solution containing goat anti-mouse IgG secondary antibody at room temperature for 1 h with gentle agitation (10 rpm).
Discard the antibody solution and wash the membrane with 10 mL of TBS solution for 5 min; repeat the washes four times.
Incubate the membrane with 2 mL of horseradish peroxidase (HRP) substrate solution for 5 min.
Remove the excess liquid from the membrane and put the membrane wrapped in plastic in an X-ray film cassette, and detect the signal using the X-ray film with an X-ray film processor.
Liposome binding assay
In this qualitative assay, tagged protein is incubated with liposomes composed of different ratios of two lipids; bound protein is pulled down with the liposomes and detected by western blotting (Figure 2 and Figure 5B). We recommend the reader to use a tag protein (e.g., GST, GFP, or MBP) or a protein without lipid-binding capacity as a negative control for the experiment.
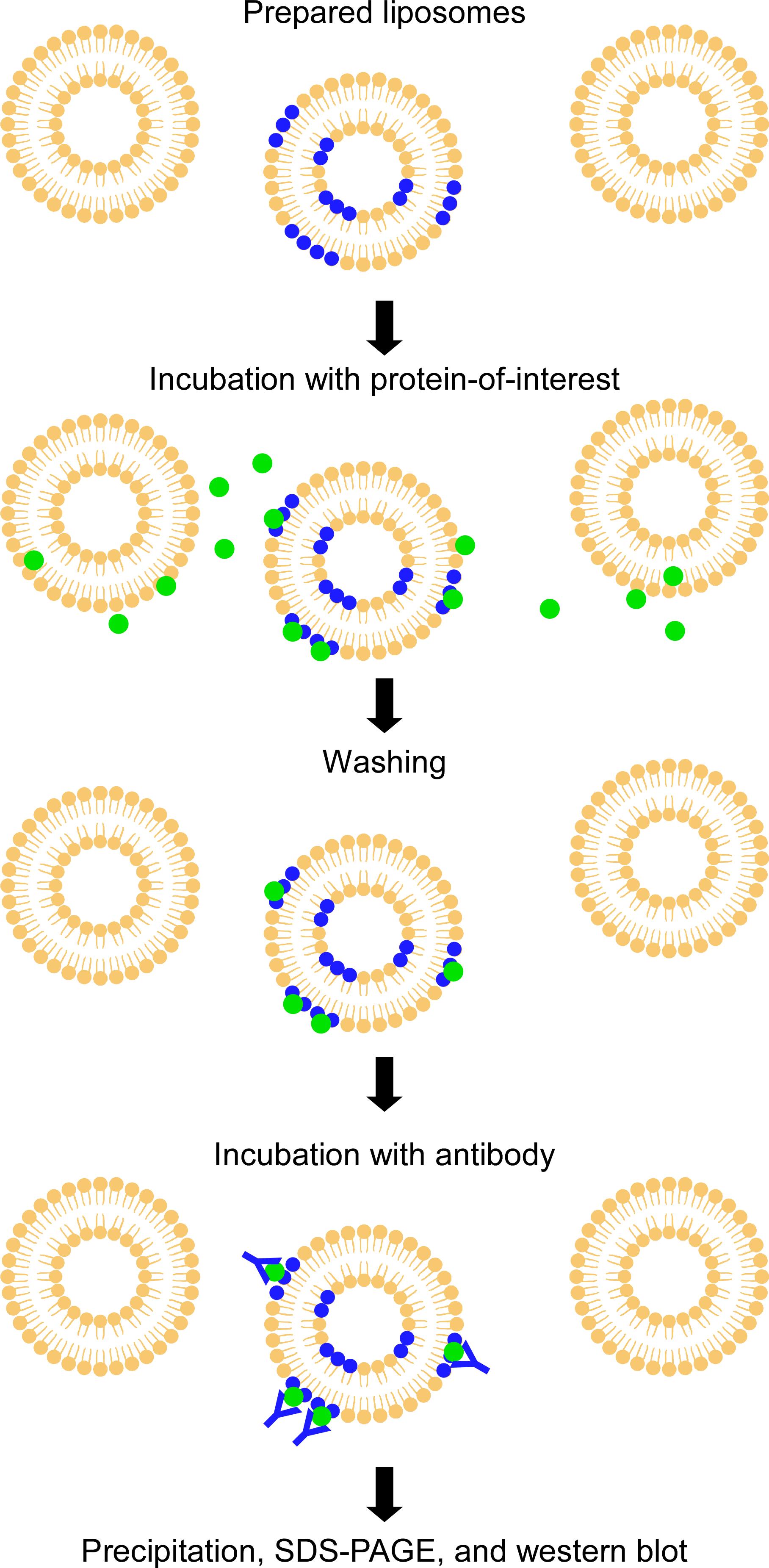
Figure 2. Liposome binding assay. A protein-of-interest is incubated with liposomes composed of different ratios of two lipids. Liposome-bound proteins are precipitated and detected by western blotting.Dissolve the lipids in pure chloroform to prepare stock solutions (1 mg/mL). Use a Volac Pasteur pipette to mix and transfer the lipid solution.
Store the lipid stock solutions in amber glass vials at -20°C.
To make the liposomes, transfer the phospholipid solutions into protein LoBind tubes in the desired ratios, with 50 µg lipids per reaction. For example, PG/PC liposome: 50/0 µg, 40/10 µg, 30/20 µg, 20/30 µg, 10/40 µg, and 0/50 µg.
Evaporate the chloroform by leaving the tube open to obtain a lipid film in each tube.
Rehydrate the lipid film with 100 µL of TBS and incubate at 37°C for 1 h.
Vortex to disperse the lipids for 10 min.
Sonicate the lipid mixture (high setting: 320W) for 10 min (30 s on and 30 s off) in an ice-water bath.
Replace the ice-water from the water bath and repeat step B7.
Centrifuge the liposomes for 10 min at 20,000 × g and 4°C.
Discard the supernatant, and wash the liposomes with 100 µL of cold TBS twice.
Resuspend the liposomes in 50 µL of cold TBS (final concentration of 1 mg/mL).
Add 1 µg of purified protein (His-FT) to the liposome solution and incubate at 30°C for 30 min with gentle agitation. Use one tube without liposomes (only TBS) as a negative control.
Centrifuge the mixture for 10 min at 20,000 × g and 4°C.
Remove the supernatant and store in another tube for western blot control.
Wash the liposome twice with 100 µL of cold TBS solution. Vortex the mixture until all the pellet is completely resuspended.
Add 10 µL of 6× SDS sample buffer to the pellet and supernatant from step B13 and incubate the sample at 95°C for 5 min in a heat block. Store the sample at -20°C until further use.
Prepare 12% standard polyacrylamide gel for SDS-PAGE (mini gel: 10 × 8 cm; 11 wells).
Load the sample into the well, run the gel with 110 V for 2 h, and transfer the protein onto a PVDF membrane.
Place the membrane in the western blot tray and incubate with 10 mL of blocking solution (TBS containing 3% [w/v] skim milk), at room temperature for 1 h with agitation (50 rpm).
Discard the blocking solution and briefly wash the membrane with ddH2O.
Incubate the membrane with the primary antibody, anti-His monoclonal antibody, in 10 mL of TBS containing 1% (w/v) bovine serum albumin at 4°C overnight.
Discard the antibody solution and wash the membrane with 10 mL of TBS solution for 5 min four times.
Incubate the membrane with the goat anti-mouse IgG secondary antibody in 10 mL of TBS containing 1% (w/v) bovine serum albumin at room temperature for 1 h.
Discard the antibody solution and wash the membrane with 10 mL of TBS solution for 5 min four times.
Incubate the membrane with 2 mL of HRP substrate solution for 5 min.
Remove the excess liquid from the membrane and put the membrane wrapped in plastic in an X-ray film cassette, and detect the signal using the X-ray film with an X-ray film processor.
Biotin-phospholipid pull-down assay
In this qualitative assay, biotinylated lipids are incubated with protein; the lipids are pulled down with streptavidin beads, and bound protein is detected by western blotting (Figure 3 and Figure 5C).
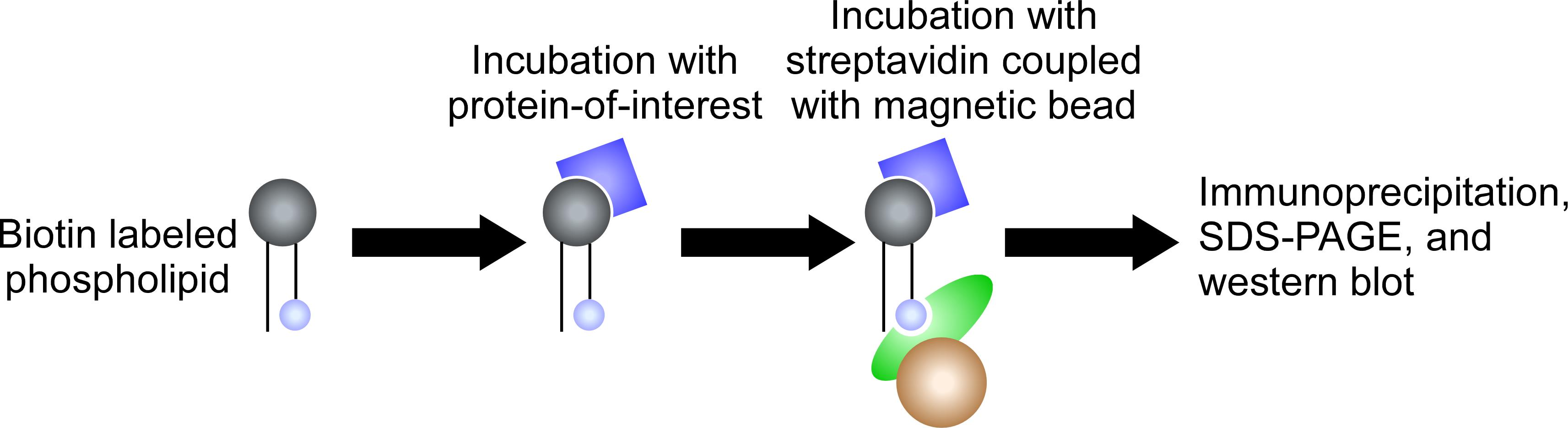
Figure 3. Biotin-phospholipid pull-down assay. Biotin-labeled phospholipids are incubated with a protein-of-interest. The protein-lipids complexes are pulled down by streptavidin beads. The lipid-bound proteins are detected by western blotting.Dissolve the biotin-labeled lipids in pure chloroform to prepare stock solutions (1 mg/mL). Use a Volac Pasteur pipette to mix and transfer the lipid solutions.
Store the lipid stock solutions in amber glass vials at -20°C.
Transfer 15 µL (15 µg) of each biotin-labeled phospholipid into a protein LoBind tube, and evaporate the chloroform to obtain a lipid film.
Rehydrate the lipid film with 10 µL of 50% ethanol.
Incubate the lipid solution at room temperature for 15 min with brief vortexing every 5 min.
Add 40 µL of TBS containing 1 µg of purified protein to the lipid solution.
Use 50 µL of TBS containing 1 µg of purified protein (without lipid solution) as a negative control.
Incubate at 30°C for 30 min with gentle agitation.
During the incubation in step C7, prepare the beads by transferring 20 µL of Dynabeads MyOne Streptavidin T1 to protein LoBind tubes.
Collect the beads with the magnetic separation rack, and wash the beads twice with 500 µL of TBS containing 0.5% (w/v) bovine serum albumin.
Resuspend the beads in 450 µL of TBS containing 0.5% (w/v) bovine serum albumin.
Spin down the lipid–protein mixture from step C7 briefly, put 5 µL of the supernatant into a new tube as an input control (10% input), and transfer the rest of the mixture into the tube containing the beads. Store the input sample at -20°C.
Incubate the bead–lipid–protein mixture in a tube rotator (10 rpm) at 4°C overnight.
Collect the beads with the magnetic separation rack and wash the beads twice with 500 µL of TBS containing 0.5% (w/v) bovine serum albumin.
Collect the beads from the last washing step and add the SDS sample buffer to the beads and the input samples from step C11. Incubate the samples at 95°C for 5 min in a heat block. Store the samples at -20°C.
Prepare a standard 12% polyacrylamide gel for SDS-PAGE.
Load the sample into the well, run the gel with 110 V for 2 h, and transfer the protein onto a PVDF membrane.
Place the membrane in the western blot tray and incubate with 10 mL of blocking solution (TBS containing 3% [w/v] skim milk) at room temperature for 1 h with gentle agitation (50 rpm).
Discard the blocking solution and briefly wash the membrane with ddH2O.
Incubate the membrane with the primary antibody, antiHis monoclonal antibody, in 10 mL of TBS containing 1% (w/v) bovine serum albumin at 4°C overnight.
Discard the antibody solution and wash the membrane with 10 mL of TBS for 5 min; repeat the washes four times.
Incubate the membrane with the goat anti-mouse IgG secondary antibody in 10 mL of TBS containing 1% (w/v) bovine serum albumin at room temperature for 1 h.
Discard the antibody solution and wash the membrane with 10 mL of TBS solution for 5 min; repeat the washes four times.
Incubate the membrane with 2 mL of HRP substrate solution for 5 min.
Remove the excess liquid from the membrane and put the membrane wrapped in plastic in an X-ray film cassette, and detect the signal using the X-ray film with an X-ray film processor.
Fluorescence polarization assay
In this quantitative assay, protein–lipid interactions alter the polarity of light emitted from a fluorophore attached to the protein of interest and excited with polarized light; graphing the resulting anisotropy values as a function of liposome concentration gives the protein–lipid binding affinity (Figure 4 and Figure 5D).
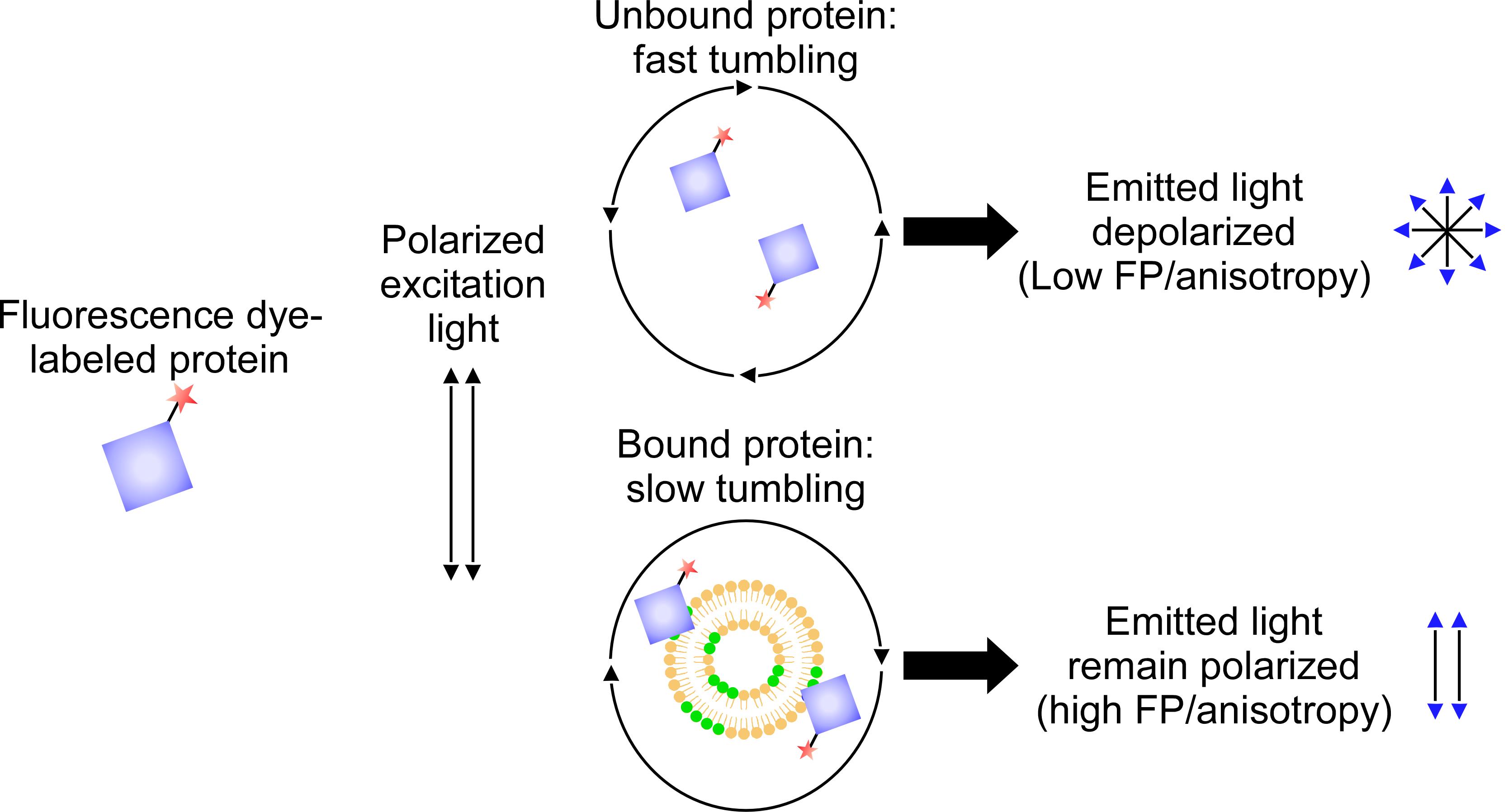
Figure 4. Fluorescence polarization assay. Fluorescence dye-labeled protein are incubated with liposomes. The degree of the interaction is calculated through the value of fluorescence polarization or anisotropy.Dissolve the lipids in pure chloroform to prepare stock solutions (1 mg/mL). Use a Volac Pasteur pipette to mix and transfer the lipid solutions.
Store the lipid stock solutions in amber glass vials at -20°C.
Prepare 1 mg of purified protein (His-FT) in 500 µL of PBS dialysis buffer. Avoid any amine molecules such as Tris or glycine in the buffer.
To label the protein, add the 500 µL of protein solution to a vial containing NHS-Rhodamine reagent from the kit and mix with pipette until all the dye dissolves.
Incubate the vial at room temperature for 1 h in the dark.
To remove the unbound dye, prepare two spin columns by resuspending the resin from the kit by vortexing and pipetting and then transfer 400 µL of resin to each spin column.
Centrifuge the columns for 30 s at 1,000 × g at room temperature to remove the buffer.
Put the column in the new collection tube and add 250 µL of protein–dye mixture from step D5 to the spin column.
Mix the sample by vortexing and pipetting.
Centrifuge the column for 30 s at 1,000 × g at room temperature.
Collect and combine the flow-through from both collection tubes.
Dilute the labeled protein mixture with PBS dialysis buffer to a concentration of 4 µM (equal to concentration of 0.1 µg/µL for His-FT) as a stock.
Aliquot and store the labeled protein in a protein LoBind tube at -20°C in the dark until further use.
To make the liposomes, transfer phospholipid solutions into microcentrifuge tubes to produce the desired concentration series, as described below. Calculate the amount of phospholipids needed prior to making stock solutions for the serial dilution analysis. Detailed information, including the molecular weight of phospholipids, can be obtained from the Avanti Polar Lipids website (https://avantilipids.com/).
In this case, we used DPPG (MW: 744.492 g/mol) and DPPC (MW: 733.562 g/mol). We used serial dilutions of lipids with concentrations of 0, 10, 20, 30, 50, 75, 100, and 150 µM in 200-µL reaction volumes with three technical replicates (from 2 mM stock solution). Therefore, we transferred 298 µL of DPPG and 293 µL of DPPC stock solutions (1 mg/mL) to the microcentrifuge tube to make 200 µL of 2 mM stock liposome.
Evaporate the chloroform to obtain a lipid film.
Rehydrate the lipid film with 200 µL of PBS and incubate at 37°C for 1 h.
Vortex the lipid dispersion for 10 min.
Sonicate the samples (high setting) twice for 10 min (30 s on and 30 s off) in an ice-water bath.
Centrifuge the liposome for 10 min at 20,000 × g and 4°C.
Discard the supernatant and wash the liposomes with 200 µL of cold PBS twice.
Resuspend the liposomes in 200 µL of cold PBS (final concentration of 2 mM).
Prepare the 96-well plate and transfer 1.5 µL of protein, liposome, and PBS to each well. Set the total volume to 200 µL (Table 1). The liposome was diluted to concentrations of 0, 10, 20, 30, 50, 75, 100, and 150 µM, as mentioned above.
Table 1. Composition of each component in the assay
Component Liposome (0 µM)
Liposome
(10 µM)
Liposome
(20 µM)
----- Liposome
(150 µM)
Protein (µL)
Liposome (µL)
PBS (µL)
1.5 µL
0 µL
198.5 µL
1.5 µL
1 µL
197.5 µL
1.5 µL
2 µL
196.5 µL
----- 1.5 µL
15 µL
183.5 µL
Incubate the plate at room temperature for 30 min with gentle agitation.
Prepare the SpectraMax Multi-Mode Plate Reader. In the software interface, set temperature to 30°C, choose end point measurement with top read, choose Costar black 96-well with no lid as the assay plate, and choose auto mix for 3 s. Finally, set the excitation wavelength to 544 nm and the emission wavelength to 575 nm, with the cut-off set to 570 nm.
Calculate the anisotropy (r) value using the software. The values can be saved to a text (.txt) file or recorded manually.
Plot the obtained data (triplicates) into the scatter plot in the GraphPad Prism 5 software, with the liposome concentrations on the x-axis and the anisotropy (r) values on the y-axis.
Analyze the data using the nonlinear regression model (one site, total binding) to obtain the dissociation constant to determine the binding affinity.
Data analysis
Representative data
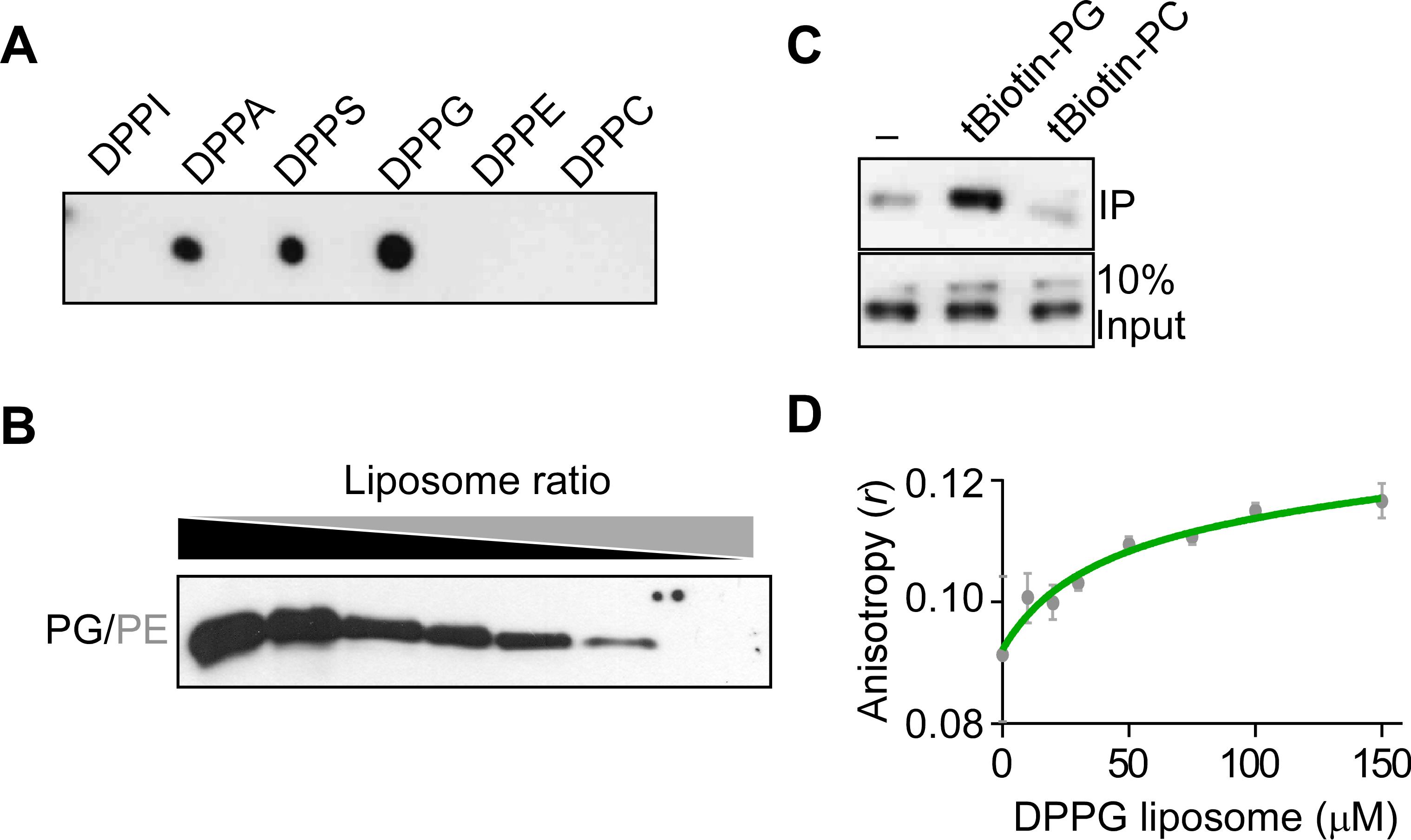
Figure 5. Representative results of in vitro FT protein–phospholipid interaction assays. A. Lipid–protein overlay assay. B. Liposome binding assay. C. Biotin-phospholipid pull-down assay. D. Fluorescence polarization assay. DPPI: dipalmitoylphosphatidylinositol, DPPA: dipalmitoylphosphatidic acid, DPPS: dipalmitoylphosphatidylserine, DPPG: dipalmitoylphosphatidylglycerol, DPPE: dipalmitoylphosphatidylethanolamine, DPPC: dipalmitoylphosphatidylcholine, PG: phosphatidylglycerol, PE: phosphatidylethanolamine, PC: phosphatidylcholine.
Recipes
The water used in the following recipes is double-distilled water (ddH2O).
Dialysis buffer (pH 8.0)
50 mM Tris
150 mM NaCl
5% glycerol
Filter sterilize and add 5 mM 2-mercaptoethanol immediately prior to use.
Tris-buffered saline (TBS) (pH 7.2)
50 mM Tris
150 mM NaCl
Filter sterilize.
4× SDS sample buffer
200 mM Tris-Cl pH 6.8
8% SDS
0.4% bromophenol blue
40% glycerol
Phosphate-buffered saline (PBS) (pH 7.4)
137 mM NaCl
2.7 mM KCl
8 mM Na2HPO4
2 mM KH2PO4
Filter sterilize.
PBS dialysis buffer (pH 7.4)
137 mM NaCl
2.7 mM KCl
8 mM Na2HPO4
2 mM KH2PO4
5% glycerol
Autoclave and add 5 mM 2-mercaptoethanol immediately prior to use.
Acknowledgments
This work was supported by a National Research Foundation (NRF) of Korea grant funded by the Korean government (NRF-2017R1A2B3009624 to J.H.A.) and Samsung Science and Technology Foundation (SSTF-BA1602-12 to J.H.A). The authors thank H.K. Song for fluorescence polarization spectroscopy experiments.
Competing interests
The authors declare no competing interests.
References
- Bernier, I., Tresca, J. P. and Jolles, P. (1986). Ligand-binding studies with a 23 kDa protein purified from bovine brain cytosol. Biochim Biophys Acta 871(1): 19-23.
- Jin, S., Nasim, Z., Susila, H. and Ahn, J. H. (2021). Evolution and functional diversification of FLOWERING LOCUS T/TERMINAL FLOWER 1 family genes in plants. Semin Cell Dev Biol 109: 20-30.
- Kim, S. C., Nusinow, D. A., Sorkin, M. L., Pruneda-Paz, J. and Wang, X. (2019). Interaction and Regulation Between Lipid Mediator Phosphatidic Acid and Circadian Clock Regulators. Plant Cell 31(2): 399-416.
- Nakamura, Y., Andres, F., Kanehara, K., Liu, Y. C., Dormann, P. and Coupland, G. (2014). Arabidopsis florigen FT binds to diurnally oscillating phospholipids that accelerate flowering. Nat Commun 5: 3553.
- Schindelin, J., Arganda-Carreras, I., Frise, E., Kaynig, V., Longair, M., Pietzsch, T., Preibisch, S., Rueden, C., Saalfeld, S., Schmid, B., et al. (2012). Fiji: an open-source platform for biological-image analysis. Nature Methods 9(7): 676-682.
- Susila, H., Juric, S., Liu, L., Gawarecka, K., Chung, K. S., Jin, S., Kim, S. J., Nasim, Z., Youn, G., Suh, M. C., Yu, H. and Ahn, J. H. (2021). Florigen sequestration in cellular membranes modulates temperature-responsive flowering. Science 373(6559): 1137-1142.
- Yao, H., Wang, G., Guo, L. and Wang, X. (2013). Phosphatidic acid interacts with a MYB transcription factor and regulates its nuclear localization and function in Arabidopsis. Plant Cell 25(12): 5030-5042.
Article Information
Publication history
Accepted: Apr 5, 2022
Published: May 20, 2022
Copyright
© 2022 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Susila, H., Jurić, S., Gawarecka, K., Chung, K. S., Jin, S., Kim, S. J., Nasim, Z., Youn, G. and Ahn, J. H. (2022). In vitro Assays to Evaluate Specificity and Affinity in Protein-phospholipid Interactions. Bio-protocol 12(10): e4421. DOI: 10.21769/BioProtoc.4421.
- Susila, H., Juric, S., Liu, L., Gawarecka, K., Chung, K. S., Jin, S., Kim, S. J., Nasim, Z., Youn, G., Suh, M. C., Yu, H. and Ahn, J. H. (2021). Florigen sequestration in cellular membranes modulates temperature-responsive flowering. Science 373(6559): 1137-1142.
Category
Plant Science > Plant biochemistry > Protein
Biochemistry > Protein > Interaction
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Tips for asking effective questions
+ Description
Write a detailed description. Include all information that will help others answer your question including experimental processes, conditions, and relevant images.
Share
Bluesky
X
Copy link







