- Submit a Protocol
- Receive Our Alerts
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Purification and Immunostaining of Mouse Ependymal Ciliary Shafts
Published: Vol 14, Iss 14, Jul 20, 2022 DOI: 10.21769/BioProtoc.4467 Views: 1739
Reviewed by: David PaulYoko BekkuNona Farbehi

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols
A Multi-color Bicistronic Biosensor to Compare the Translation Dynamics of Different Open Reading Frames at Single-molecule Resolution in Live Cells
Amanda L. Koch [...] Timothy J. Stasevich
Jul 20, 2021 2345 Views
Purification and Cryo-electron Microscopy Analysis of Plant Mitochondrial Ribosomes
Florent Waltz [...] Yaser Hashem
Aug 5, 2021 2257 Views
Immunoprecipitation of Reporter Nascent Chains from Active Ribosomes to Study Translation Efficiency
Roberta Cacioppo and Catherine Lindon
Sep 20, 2023 372 Views
Abstract
Cilia and flagella are microtubule-based hair-like organelles protruding from the surface of most eukaryotic cells, and play essential roles in cell locomotion, left-right asymmetry, embryo development, and tissue homeostasis. With isolated cilia and flagella, great progress has been made in understanding the composition, structure, and function of cilia. However, the current cilia/flagella isolation methods are deficient in the integrity or productivity of purified cilia when applied to mammalian motile cilia. Here, we describe a new protocol that isolates cilia shafts from mouse ependymal cells, by horizontal shear force and mild detergent. This method enables the production of virtually integral cilia with high yields and less cell body contamination. It is suitable for immunostaining, puromycin labeling assay, and proximity ligation assay of mammalian motile cilia.
Graphical abstract:
Background
Multiple cilia/flagella isolation methods, including pH-shock, calcium-shock, dibucaine treatment, and mechanical procedures (e.g., peel-off, and slide-pull), have been developed in various organisms (Witman et al., 1972, 1978, 1986; Adoutte et al., 1980; Mitchell et al., 2009). The pH- and calcium-shock methods were first created to purify cilia from Euglena and Tetrahymena, respectively (Gibbons, 1965; Rosenbaum and Child, 1967). Later, the two methods became the most used cilia/flagella purification methods in protozoa. In mammals, mechanical peel-off or slide-pull approaches have been used to isolate primary cilia from cultured cells, such as MDCK (Madin-Darby canine kidney) cells, normal mouse cholangiocytes (NMCs), and normal rat cholangiocytes (NRCs) (Huang et al., 2006; Kiesel et al., 2020). The calcium-shock method combined with mechanical agitation is used to separate motile cilia from rabbit oviduct, pig trachea, and primary cultured mouse ependymal cells (mEPCs) (Anderson, 1974; Hastie et al., 1986; Zheng et al., 2019). It is also used to isolate primary cilia from rat olfactory cilia and inner medullary collecting duct (IMCD3) cells (Mayer et al., 2008; Ishikawa et al., 2012). The ciliary protein composition and high-resolution ciliary ultrastructure have been well studied using these methods in purified cilia/flagella. However, these procedures usually cause cilia membrane damage and ignore the physiological activity of isolated cilia, thus hindering further exploration of cilia biology.
Here, we report a protocol of cilia isolation, which is improved from the calcium-shock combined mechanical agitation method. To maintain the integrity of isolated cilia and reduce cytoplasmic contamination, we decreased the detergent concentration in the deciliation buffer and replaced the vortex with horizontal shear force. With this method, we obtained a sufficient yield of motile cilia for immunostaining, puromycin labeling, and proximity ligation assays. As an example of its application, we applied this procedure to purify mouse ependymal cilia to verify ciliary RNA local translation. In addition, this method can also be used to purify motile cilia from mouse brain lateral ventricle.
Materials and Reagents
0.22 μm filter (Millipore, catalog number: SLGPR33RB)
75 cm2 Flask (Corning, catalog number: 430641)
15 mL tube (Falcon, catalog number: 352095)
12 mm diameter circle coverslips (Marienfeld, catalog number: MAR0111520)
Glass slides (Premiere, catalog number: 9308W)
24-well plate (Costar, catalog number: 3524)
15 cm Petri dish (Corning, catalog number: 430599)
Filter paper (GE, catalog number: 10311611)
Parafilm (Bemis, catalog number: PM-996)
Milli-Q water
Fibronectin (1 mg/mL) (Sigma-Aldrich, catalog number: FC010), store at 4°C
Papain (Worthington Biochemical Corporation, LS003126), store at 4°C
DMEM (ThermoFisher Scientific, GibcoTM, catalog number:12430054), store at 4°C
Primocin (50 mg/mL) (InvivoGen, ant-pm-2), store at -20°C
Fetal bovine serum (Ausbian, VS500T), store at -20°C
Poly-L-lysine hydrobromide (Sigma-Aldrich, catalog number: P1399), store the powder at -20°C.
Note: Sterilize the stock solution (100 mg/mL Poly-L-lysine in Milli-Q water) with a 0.22 μm filter, and store it in aliquots at -20°C.
Trition X-100 (Sangon Biotech, catalog number: A110694)
Tween 20 (Sangon Biotech, catalog number: A100777)
Paraformaldehyde (PFA) (Sigma-Aldrich, catalog number: P6148), store the powder at 4°C
Bovine Serum Albumin (BSA) (Sigma-Aldrich, catalog number: A3912), store the powder at 4°C
Mouse anti-Acetylated Tubulin (Sigma-Aldrich, catalog number: T6793), store at 4°C
Rabbit anti-Cep290 (home-made) (Zhao et al., 2021), store at -20°C
Guinea pig anti-Odf2 (home-made) (Zhao et al., 2019), store at -20°C
Donkey anti-mouse IgG conjugated with Alexa Fluor 488 (ThermoFisher Scientific, catalog number: A-21202), long-term storage at -80°C, short-term storage at 4°C
Donkey anti-rabbit IgG conjugated with Cy3 (Jackson ImmunoResearch, catalog number: 711-165-152), long-term storage at -80°C, short-term storage at 4°C
Donkey anti-guinea pig IgG conjugated with Alexa Fluor 647 (Jackson ImmunoResearch, catalog number: 706-605-148), long-term storage at -80°C, short-term storage at 4°C
ProLong Diamond Antifade Mountant (ThermoFisher Scientific, catalog number: P36970), store at 4°C
Immersion oil (Lecia microsystem, catalog number: 12847995)
PBS (see Recipes), store at 4°C
Dissection buffer (see Recipes), store at 4°C
Enzymatic digestion buffer (see Recipes), freshly prepared
mEPC culture medium (see Recipes), store at 4°C
Starvation culture medium (see Recipes), store at 4°C
Deciliation buffer (see Recipes), store at 4°C
4% PFA (see Recipes)
TBST (10×) (see Recipes)
Blocking buffer (see Recipes), stored in aliquots at -20°C
Equipment
Milli-Q (Millipore, model: Advantage A10)
Sharp tweezer (Dumont, catalog number: 0208-5/45-PO)
Tweezer (Dumont, catalog number: 0203-5/15-PO)
Horizontal shaker (Zhichu, model: ZQZY-AS9)
Superspeed centrifuge (ThermoFisher Scientific, model: Sorvall LYNX 6000, catalog number:75006590)
Swinging bucket rotor (ThermoFisher Scientific, model: BIOFlexTM HC, catalog number: 75003000)
Confocal microscopy (Leica, model: TCS SP8 WLL system equipped with a 63×/1.4 oil immersion objective)
Procedure
Preparation before ciliary shaft purification
Grow and maintain mouse ependymal cells (mEPCs) in a 75 cm2 flask (Hao et al., 2021).
Add 6 mL of fibronectin (10 μg/mL in PBS) to a 75 cm2 flask, and incubate at 37°C for 24 h to coat the flask the day before culture.
Dissect the telencephala from five C57BL/6 mouse pups at postnatal day 0 to 1 (P0–P1) in ice-cold dissection buffer, and remove the hippocampus, the cerebellum, the choroid plexus, the olfactory bulb, and meninges with sharp tweezers.
Transfer the dissected telencephala into a 15-mL tube, and digest them with 10 mL of freshly prepared enzymatic digestion buffer at 37°C for 30 min.
Dissociate cells carefully by pipetting ten times with a 5-mL pipette, and collect cells by centrifugation at 400 × g at room temperature for 5 min.
Resuspend cells with mEPC culture medium, and seed them into a 75-cm2 fibronectin-coated flask.
Shake off and remove neurons one day after seeding.
When cells are confluent, rinse the cells with PBS, and add 10 mL of starvation culture medium to induce differentiation.
Harvest cells after culturing for ten days in starvation culture medium.
Note: The ratio of multiciliated cells should be approximately 50%, when harvested on day 10 of serum starvation (Figure 1).
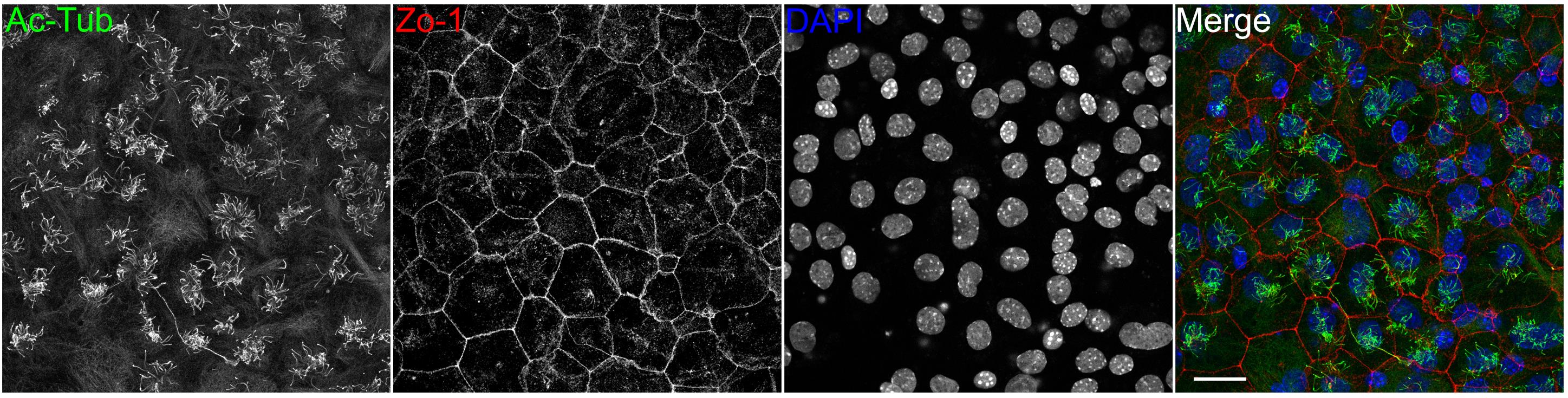
Figure 1. Primary cultured mouse ependymal cells. Acetylated tubulin (Ac-tub) decorates ciliary axonemes. Zo-1 and DAPI label tight junctions and nuclei, respectively. Scale bar, 25 μm.Coat coverslips with poly-L-lysine, one day before ciliary shaft purification.
Place 12 sterilized coverslips in a 24-well plate, with one coverslip per well.
Rinse coverslips three times, with 500 μL of PBS per well.
Add 500 μL of poly-L-lysine in PBS (100 ng/μL) to each well, and incubate at 37°C for 24 h.
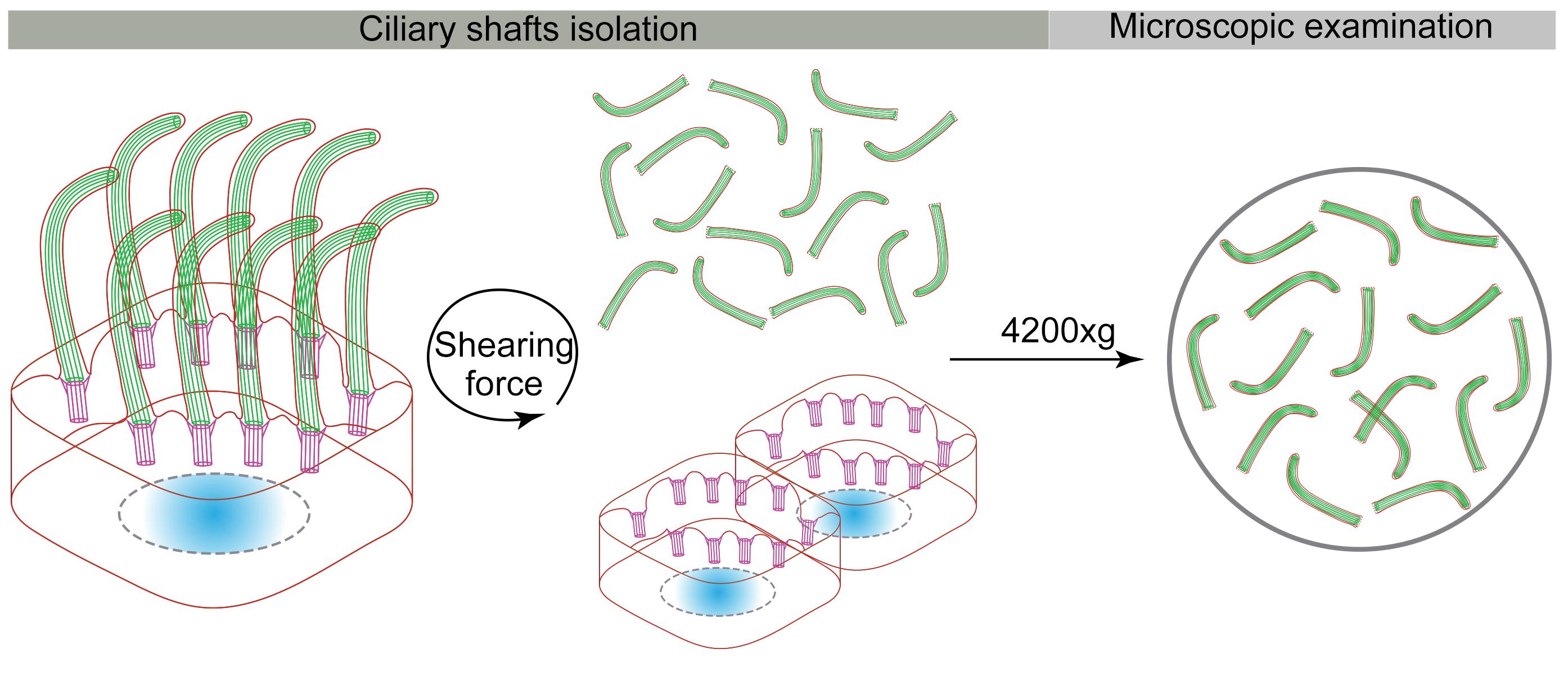
Ciliary shaft purification
Wash mEPCs twice with 6 mL of ice-cold PBS, and twice with 6 mL of ice-cold deciliation buffer quickly.
Into the flask, add 9 mL of ice-cold deciliation buffer containing 0.01% Triton X-100.
Fix the flask on a horizontal shaker with a sticky green rubber base (Figure 2).
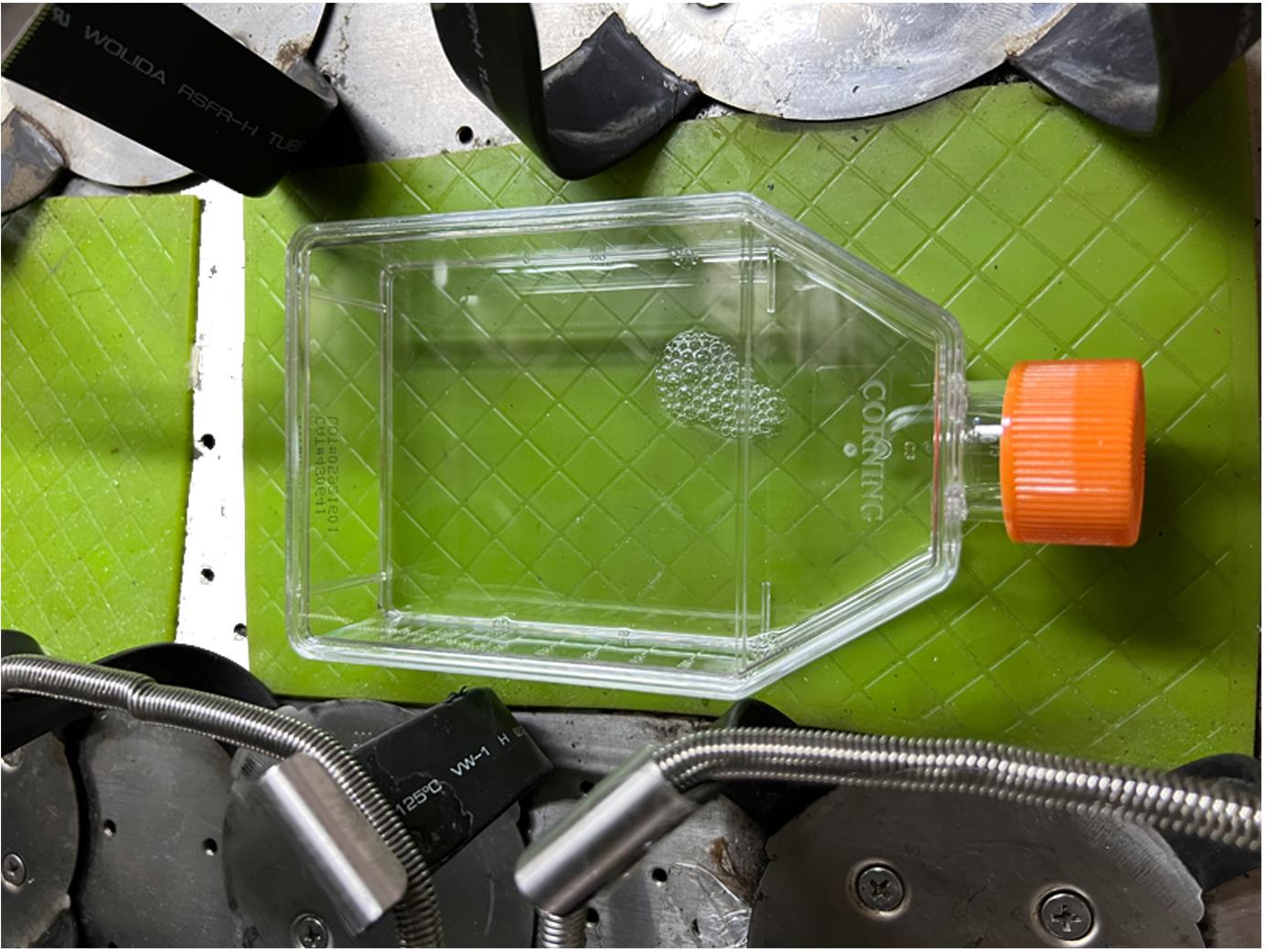
Figure 2. Representative image of an adhered flask on a horizontal shaker.Immediately shake at 300 rpm and 37°C for 15 min, to detach cilia from the base of the transition zone (Video 1).
Video 1. Ciliary shaft purification using shear force.Transfer the supernatant containing ciliary shafts into a 15-mL tube.
Centrifuge horizontally in a swinging bucket rotor (BIOFlexTM HC) at 600 × g and 4°C for 10 min to remove cell debris.
Rinse the poly-L-lysine coated coverslips three times with 500 μL of PBS per well.
Aliquot the supernatant into 12 wells with poly-L-lysine coated coverslips in equal amounts (720 μL of supernatant per well).
Place the 24-well plate in a swinging bucket rotor (BIOFlexTM HC).
Centrifuge horizontally at 4,200 × g and 4°C for 10 min.
Wash the coverslips once with 500 μL of PBS per well.
Now, the purified ciliary shafts are ready for immunostaining, puromycin labeling, and proximity ligation assays.
Note: It is recommended to use immunofluorescence staining to examine the quality and integrity of cilia. The antibodies against Arl13b (a ciliary membrane protein) and acetylated tubulin are for the quality and integrity of ciliary membrane and axonemes, respectively. The antibodies against Cep290 (a transition zone protein) and Odf2 (a basal body protein) are used to check if the purified ciliary shafts contain the transition and lack the basal body.
Immunostaining of purified ciliary shafts
Fix the ciliary shafts with 500 μL of 4% PFA per well at room temperature for 15 min.
Briefly wash with 500 μL of PBS per well.
Permeabilize with 500 μL of 0.5% Triton X-100 in PBS per well for 15 min.
Briefly wash with 500 μL of PBS per well.
Incubate with 500 μL of blocking buffer (4% BSA in TBST) per well at room temperature for 1 h.
Make a humidity chamber (Figure 3).
Wrap the outer surface of a 15 cm Petri dish with tin foil, to protect the sample from light. Place the shiny side of the tinfoil facing out of the humidity chamber.
Place a water-soaked filter paper into the Petri dish.
Place a piece of parafilm on the filter paper.
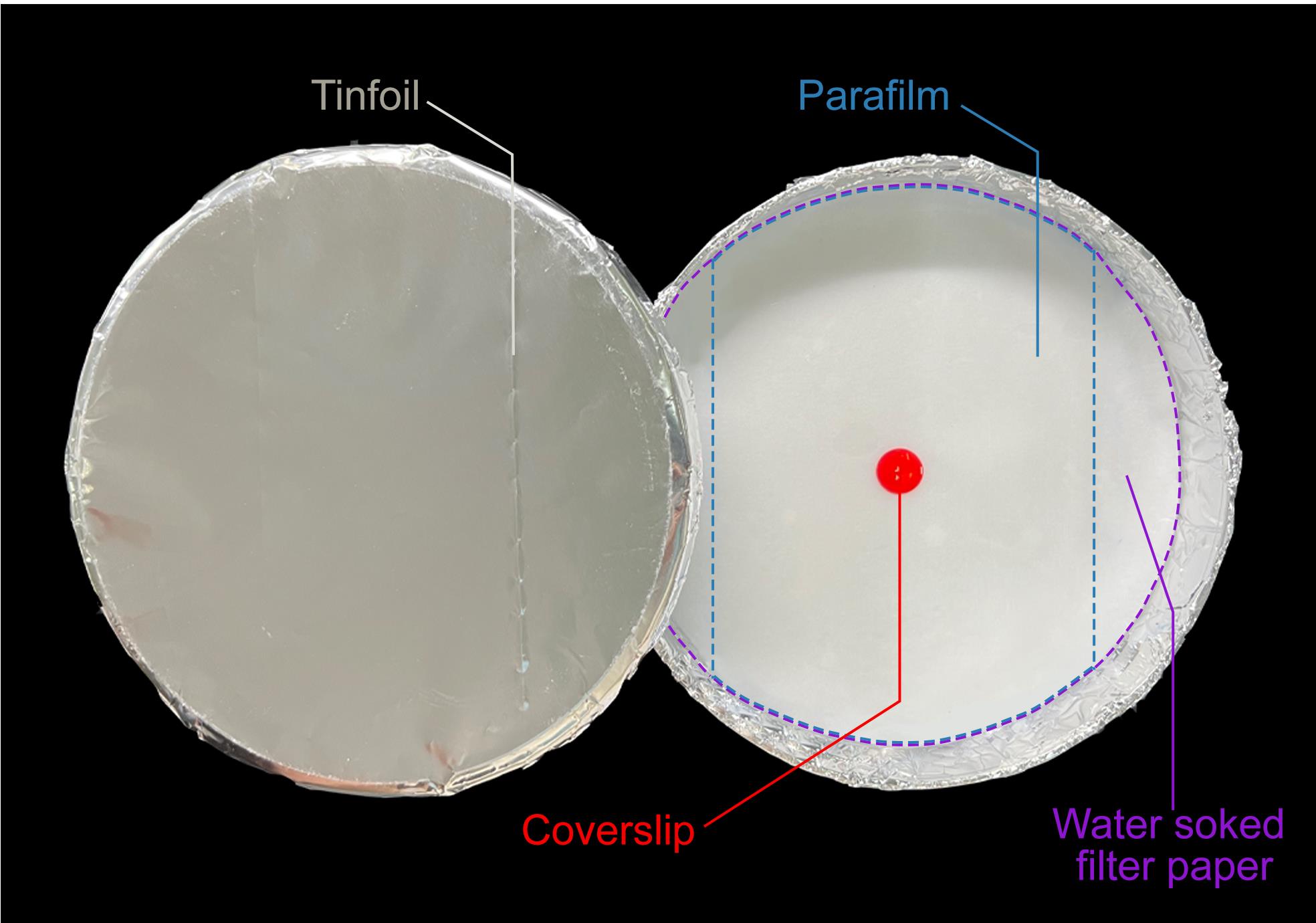
Figure 3. Representative image of a humidity chamber. Tinfoil, filter paper, parafilm, and coverslip are noted. Filter paper and parafilm are outlined by purple and blue dashed lines, respectively.Using a tweezer, transfer the coverslips onto the parafilm. Place the side with ciliary shafts upward.
Incubate each coverslip with 90 μL of primary antibodies diluted in blocking buffer at 4°C overnight.
Wash with 200 μL of blocking buffer three times for 5 min each.
Incubate each coverslip with 90 μL of secondary antibodies diluted in blocking buffer at room temperature for 1 h.
Wash with 200 μL of blocking buffer three times for 5 min each.
Briefly wash with 200 μL of Milli-Q water per coverslip.
Drop 10 μL of mounting medium (ProLong Diamond Antifade Mountant) per slide.
Remove excess Milli-Q water from coverslips with a filter paper.
Mount the coverslips onto the slides immediately (Cao et al., 2014).
Dry the sample at room temperature for at least 2 h in the dark, and store it at 4°C.
Imaging of purified ciliary shafts by confocal microscope
Confocal microscopy images were taken on a Leica TCS SP8 WLL system with a 63×/1.4 oil immersion objective. Immersion oil with a refractive index of 1.518 was used to minimize spherical aberrations. Scan speed was 400 Hz, line average was 3, and optical sections were captured at 0.5-μm intervals along the z-axis. A typical image is shown in Figure 4. For extra fluorescence images, please refer to the supplementary Figure 2C of the original research article (Hao et al., 2021).
Measurement parameters for confocal imaging are in Table 1, and proper ciliary shaft density for fluorescence imaging is shown in Figure 4.
Table 1. Filters and measurements parameters
Filter Excitation Emission Laser (nm) Intensity Detector Wavelength (nm) Gain Alexa Fluor 488 498 1.5% HyD standard mode 508–542 81% Cy3 550 7% HyD standard mode 560–620 100% Alexa Fluor 647 650 2% HyD standard mode 660–720 80% 
Figure 4. Typical images of isolated ciliary shafts. Purified ciliary shafts from one 75 cm2 flask of mPECs were equally spun onto 12 pieces of 12-mm coverslips. Ac-tub decorates ciliary axonemes. Cep290 marks the transition zone. Scale bar, 25 μm.
Recipes
PBS, pH 7.4
Reagent Final concentration Amount NaCl 137 mM 8 g KCl 2.7 mM 0.2 g KH2PO4 2 mM 0.24 g Na2HPO4 10 mM 1.44 g Milli-Q water n/a 1,000 mL Total n/a 1,000 mL mEPC culture medium
Reagent Final concentration Amount Primocin 50 μg/mL 500 μL DMEM n/a 500 mL FBS 10% 50 mL Total n/a 500 mL Starvation culture medium
Reagent Final concentration Amount Primocin 50 μg/mL 500 μL DMEM n/a 500 mL Total n/a 500 mL Dissection buffer, pH 7.4
Reagent Final concentration Amount NaCl 161 mM 9.4 g KCl 5 mM 0.37 g MgSO4 1 mM 0.12 g CaCl2 4.7 mM 0.41 g HEPES 5 mM 1.2 g Glucose 5.5 mM 0.99 g Milli-Q water n/a 1,000 mL T0tal n/a 1,000 mL Enzymatic digestion buffer
Reagent Final concentration Amount EDTA (50mM stock) 0.5 mM 100 μL CaCl2 (100mM stock) 1 mM 100 μL NaOH (1M stock) 1.5 mM 15 μL Papain 10 U/mL 100 U Dissection buffer n/a 10 mL Total n/a 10 mL Deciliation buffer, pH 5.5
Reagent Final concentration Amount Sucrose 250 mM 85.575 g CaCl2 20 mM 2.22 g Pipes 20 mM 6.047 g Milli-Q water n/a 1,000 mL Total n/a 1,000 mL Adjust the pH with saturated HCl. Filter-sterilize with a 0.22 μm filter, and store at 4°C.
4% PFA
Reagent Final concentration Amount Paraformaldehyde 4% 4 g PBS n/a 100 mL Total n/a 100 mL Heat the solution to 60°C with stirring, to accelerate the dissolution of PFA.
Freshly prepare, and filter-sterilize with a 0.22 μm filter before use.
TBST (10×), pH 7.5
Reagent Final concentration Amount NaCl 1.5 M 88 g Tris base 0.5 M 60 g Tween 20 0.5% 5 mL Milli-Q water n/a 1,000 mL Total n/a 1,000 mL Heat the solution to 60°C with stirring, to accelerate the dissolution of solutes.
Blocking buffer
Reagent Final concentration Amount BSA 4% 4 g TBST (10×) 1× 10 mL Milli-Q wate n/a 90 mL Total n/a 100 mL Filter-sterilize with a 0.22 μm filter, and store at -20°C.
Acknowledgments
Thanks to the financial support from National Natural Science Foundation of China (31991192 for X.Z. and 31970652 for X.Y.), National Key R&D Program of China (2017YFA0503500 for X.Z. and X.Y.), and Chinese Academy of Sciences (XDB19020102 for X.Z.). Thanks to the work of Dr. Anderson (University of Texas Southwestern Medical School) for the original buffer recipe for this ciliary shaft purification method. Thanks to the work of Dr. Yawen Chen (Shanghai Institute of Biochemistry and Cell Biology), which adapted Anderson’s method to primary cultured mEPCs.
Competing interests
The authors declare no competing interests.
Ethics
Experiments involving mouse tissues were performed in accordance with protocols approved by the Institutional Animal Care and Use Committee of CAS Center for Excellence in molecular Cell Science, Shanghai Institute of Biochemistry and Cell Biology, Chinese academy of Sciences. The approval ID is SIBCB-S302-2110-037, and the validity period is from Aug 2nd 2021 to Aug 1st 2023.
References
- Adoutte, A., Ramanathan, R., Lewis, R. M., Dute, R. R., Ling, K. Y., Kung, C. and Nelson, D. L. (1980). Biochemical studies of the excitable membrane of Paramecium tetraurelia. III. Proteins of cilia and ciliary membranes. J Cell Biol 84(3): 717-738.
- Anderson, R. G. (1974). Isolation of ciliated or unciliated basal bodies from the rabbit oviduct. J Cell Biol 60(2): 393-404.
- Cao, J., Zhu, X. and Yan, X. (2014). Fluorescence Microscopy for Cilia in Cultured Cells and Zebrafish Embryos. Bio-protocol 4(14): e1188.
- Gibbons, I. R. (1965). Chemical dissection of cilia. Arch Biol (Liege) 76(2): 317-352.
- Hao, K., Chen, Y., Yan, X. and Zhu, X. (2021). Cilia locally synthesize proteins to sustain their ultrastructure and functions. Nat Commun 12(1): 6971.
- Hastie, A. T., Dicker, D. T., Hingley, S. T., Kueppers, F., Higgins, M. L. and Weinbaum, G. (1986). Isolation of cilia from porcine tracheal epithelium and extraction of dynein arms. Cell Motil Cytoskeleton 6(1): 25-34.
- Huang, B. Q., Masyuk, T. V., Muff, M. A., Tietz, P. S., Masyuk, A. I. and Larusso, N. F. (2006). Isolation and characterization of cholangiocyte primary cilia. Am J Physiol Gastrointest Liver Physiol 291(3): G500-509.
- Ishikawa, H., Thompson, J., Yates, J. R., 3rd and Marshall, W. F. (2012). Proteomic analysis of mammalian primary cilia. Curr Biol 22(5): 414-419.
- Kiesel, P., Alvarez Viar, G., Tsoy, N., Maraspini, R., Gorilak, P., Varga, V., Honigmann, A. and Pigino, G. (2020). The molecular structure of mammalian primary cilia revealed by cryo-electron tomography. Nat Struct Mol Biol 27(12): 1115-1124.
- Mayer, U., Ungerer, N., Klimmeck, D., Warnken, U., Schnolzer, M., Frings, S. and Mohrlen, F. (2008). Proteomic analysis of a membrane preparation from rat olfactory sensory cilia. Chem Senses 33(2): 145-162.
- Mitchell, K. A., Szabo, G. and Otero Ade, S. (2009). Methods for the isolation of sensory and primary cilia--an overview. Methods Cell Biol 94: 87-101.
- Rosenbaum, J. L. and Child, F. M. (1967). Flagellar regeneration in protozoan flagellates. J Cell Biol 34(1): 345-364.
- Witman, G. B. (1986). Isolation of Chlamydomonas flagella and flagellar axonemes. Methods Enzymol 134: 280-290.
- Witman, G. B., Carlson, K., Berliner, J. and Rosenbaum, J. L. (1972). Chlamydomonas flagella. I. Isolation and electrophoretic analysis of microtubules, matrix, membranes, and mastigonemes. J Cell Biol 54(3): 507-539.
- Witman, G. B., Plummer, J. and Sander, G. (1978). Chlamydomonas flagellar mutants lacking radial spokes and central tubules. Structure, composition, and function of specific axonemal components. J Cell Biol 76(3): 729-747.
- Zhao, H., Chen, Q., Fang, C., Huang, Q., Zhou, J., Yan, X. and Zhu, X. (2019). Parental centrioles are dispensable for deuterosome formation and function during basal body amplification. EMBO Rep 20(4).
- Zhao, H., Chen, Q., Li, F., Cui, L., Xie, L., Huang, Q., Liang, X., Zhou, J., Yan, X. and Zhu, X. (2021). Fibrogranular materials function as organizers to ensure the fidelity of multiciliary assembly. Nat Commun 12(1): 1273.
- Zheng, J., Liu, H., Zhu, L., Chen, Y., Zhao, H., Zhang, W., Li, F., Xie, L., Yan, X. and Zhu, X. (2019). Microtubule-bundling protein Spef1 enables mammalian ciliary central apparatus formation. J Mol Cell Biol 11(1): 67-77.
Article Information
Publication history
Accepted: May 31, 2022
Published: Jul 20, 2022
Copyright
© 2022 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Hao, K., Zhu, X. and Yan, X. (2024). Purification and Immunostaining of Mouse Ependymal Ciliary Shafts. Bio-protocol 14(14): e4467. DOI: 10.21769/BioProtoc.4467.
Category
Cell Biology > Organelle isolation
Molecular Biology > RNA > mRNA translation
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Tips for asking effective questions
+ Description
Write a detailed description. Include all information that will help others answer your question including experimental processes, conditions, and relevant images.
Share
Bluesky
X
Copy link









