- Submit a Protocol
- Receive Our Alerts
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Maximizing the Rod Outer Segment Yield in Retinas Extracted from Cattle Eyes
Published: Vol 14, Iss 14, Jul 20, 2022 DOI: 10.21769/BioProtoc.4474 Views: 1044
Reviewed by: Gal HaimovichSrinidhi Rao Sripathy RaoAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols
E15.5 Mouse Embryo Micro-CT Using a Bruker Skyscan 1172 Micro-CT
Elena Astanina [...] Federico Bussolino
May 5, 2023 310 Views
THRIFTY—A High-throughput Single Muscle Fiber Typing Method Based on Immunofluorescence Detection
Sebastian Edman [...] William Apró
May 20, 2023 397 Views
Princeton RAtlas: A Common Coordinate Framework for Fully cleared, Whole Rattus norvegicus Brains
Emily Jane Dennis [...] Carlos D. Brody
Oct 20, 2023 567 Views
Abstract
The retina is a thin neuronal multilayer responsible for the detection of visual information. The first step in visual transduction occurs in the photoreceptor outer segment. The studies on photoreception and visual biochemistry have often utilized rod outer segments (OS) or OS disks purified from mammalian eyes. Literature reports several OS and disk purification procedures that rarely specify the procedure utilized to collect the retina from the eye. Some reports suggest the use of scissors, while others do not mention the issue as they declare to utilize frozen retinas. Because the OS are deeply embedded in the retinal pigmented epithelium (RPE), the detachment of the retina by a harsh pull-out can cause the fracture of the photoreceptor cilium. Here, we present a protocol maximizing OS yield. Eye semi-cups, obtained by hemisecting the eyeball and discarding the anterior chamber structures and the vitreous, are filled with Mammalian Ringer. After 10–15 min of incubation, the retinas spontaneously detach with their wealth of OS almost intact. The impressive ability of the present protocol to minimize the number of OS stuck inside the RPE, and therefore lost, compared with the classic procedure, is shown by confocal laser scanning microscopy analysis of samples stained ex vivo with a dye (MitoTracker deep red) that stains both retinal mitochondria and OS. Total protein assay of OS disks purified by either procedure also shows a 300% total protein yield improvement. The advantage of the protocol presented is its higher yield of photoreceptor OS for subsequent purification procedures, while maintaining the physiological features of the retina.
Keywords: BovineBackground
The vertebrate eye is a photoreceptive sense organ (Stenkamp, 2015). Its laminar structure comprises three main layers: the fibrous tunic, consisting of the cornea and sclera; the vascular tunic (uvea), including the iris, ciliary body, and choroid; and the nervous tunic, including the retina and the retinal pigmented epithelium (RPE) (Netter, 2018). The eye can also be divided into an anterior and a posterior segment. The former includes the cornea, iris, ciliary body, and lens, while the latter encompasses the vitreous, retina, RPE, and choroid. The RPE faces the sensory retina and separates the subretinal space from the choroid, forming the outer blood–retinal barrier. Aqueous humor fills the anterior segment. The mammalian retina, a part of the central nervous system, consists of five classes of neurons (retinal ganglion cells, amacrine cells, bipolar cells, horizontal cells, and the cone and rod photoreceptors), arranged into three nuclear and two synaptic layers (Masland, 2012). The input for visual signaling (Palczewski, 2014) comes from photoreceptor cells, distinct in cones, responsible for the chromatic vision, and rods, high-sensitive receptors responsible for scotopic vision, that consist of a specialized inner and outer segment. Most mammalian retinas express one short wavelength-sensitive and one long wavelength type of cone, while a few express three (Masland, 2012). A cilium connects the OS to the inner segment (IS) (Gilliam et al., 2012); in fact, rod and cone OS are structurally homologous to non-motile cilia. The OS captures photons, initiating the visual transduction (Palczewski, 2014). In particular, the rod OS consists of a stack of approximately 2,000 membranous disks surrounded by a plasma membrane, which express the proteins devoted to phototransduction (Molday and Moritz, 2015). Recently, it was reported that the proteins of the mitochondrial redox chain are also functionally expressed in the rod OS (Bruschi et al., 2020). Disks are synthesized at the base of the OS (Young, 1967) and are shed at the apical tip, where the RPE phagocytizes exhausted disks (Campbell et al., 2018). Since the 1970s, studies on photoreception and on the biochemistry of vision have typically utilized rod outer segments (OS) or OS disks from cattle eyes, as these allow to obtain a large quantity of sample. Literature reports several rod OS purification procedures (de Grip et al., 1972; Raubach et al., 1974; McDowell and Kühn, 1977; Papermaster, 1982; Zimmerman and Godchaux, 1982; Molday et al., 1987; Uhl et al., 1987; Bubis, 1998), and one isolation procedure for disks (Smith et al., 1975, 1982). However, these seldom specify the procedure utilized to collect the retina from the eye. Some reports suggest the use of scissors; others do not include this passage, as they utilize frozen retinas. Because the OS are deeply embedded in the RPE, the detachment of the retina from the RPE by a harsh procedure can cause the fracture of the photoreceptor cilium. The goal of this protocol is to describe a gentle procedure for the extraction of living retinas from bovine eyeballs, maximizing the yield of rod OS, and avoiding the fracture of the rod at the cilium level inside the RPE and their eventual loss when the eye semi-cup is discarded.
The best yield of rod OS or disks is obtained when retina is let to spontaneously detach from the RPE by filling the eye semi-cup with sterile filtered mammalian ringer (MR) for at least 15 min. In the first step of the protocol, cattle eyes, freed from periocular muscle and connective tissue, are cut at the level of the ora serrata on a section plane below the pupil, as shown in Figure 1, and the anterior portion of the eye is discarded. In this way, it is possible to eliminate the lens, the aqueous humor, and the vitreous humor, obtaining a semi-cup containing the retina (Figure 1). Then, to facilitate the spontaneous detachment of the retina, the semi-cup is filled with sterile filtered MR and incubated for at least 10 min: in this way, most of the rod OS remain attached to the free-floating retina that detaches from the RPE. By contrast, if the retina is extracted utilizing a tweezer or a soft brush, the photoreceptors can fracture at the cilium and remain immersed in the RPE. The comparison between the two methods is shown by data obtained from the confocal laser scanning microscope (CLSM) analysis shown in Figures 3 and 4, after staining the retina with MitoTracker Deep Red 633 (ThermoFisher), as previously reported (Calzia et al., 2010; Panfoli et al., 2010; Ravera et al., 2007). Specifically, the retina detached by the MR incubation method presents a homogeneous layer of photoreceptors (Figure 3, Panel A) while, in the remaining semi-cup of the eye, only mitochondria are visible (Figure 3, Panel B). By contrast, when the retina is detached with a soft brush, few recognizable OS are observed (Figure 4, Panel A), which are instead still partially stained inside the eye semi-cup (Figure 4, Panel B).

Figure 1. Scheme for the cut of the eye semi-cup. The drawing shows where the plane of section passes to obtain the eye semi-cup. The panel on the right shows a close-up of an eye semi-cup unscrewed by the crystalline lens, aqueous humor, and vitreous humor, in which the retina lining the bottom of the eye is recognized.
The innovation of the present protocol, with respect to the literature, is the use of a gentle procedure to extract the retinas from the eye semi-cup, maximizing the absolute amount of rod OS still attached to the retina. Confocal laser scanning microscopy (Figures 3 and 4) demonstrated the advantage our protocol for the extraction of the retinas offers, with respect to the conventional extraction of the retina by means of a tweezer or even using a soft brush, and the actual risk of leaving almost all the rod OS inside the RPE of the eye semi-cup (Figure 3).
The significance of the improvement represented by the present protocol is the possibility to maximize the presence of the photoreceptor OS in the retinas extracted from large mammalian eye semi-cups. This is advantageous if retinas are used to purify rod OS or disks, but also for any subsequent procedure requiring the presence of photoreceptors in the isolated retina.
Materials and Reagents
Eppendorf Tube (Eppendorf, catalog number: 0030 102.002)
Glass Pasteur Pipettes (Teklab Limited, UK catalog number: GP225)
Centrifuge tube, conical bottom, 13 mL volume (VWR, catalog number: VWRI525-0179)
Milli-Q® Biocel System water (Millipore, http://www.millipore.com/)
Ampicillin (Millipore, catalog number: 171257), storage at -20°C
Protease inhibitor cocktail (Millipore, catalog number: 539133), storage at -20°C
NaCl (VWR, catalog number: 7647-14-5), room temperature storage
KCl (VWR, catalog number: 7447-40-7), room temperature storage
Na2HPO4 (Millipore, catalog number: 567547), room temperature storage
NaH2PO4 (Millipore, catalog number: 567545), room temperature storage
MgCl2·6H2O (Millipore, catalog number: 442615), room temperature storage
CaCl2·2H2O (Millipore, catalog number: 137101), room temperature storage
Steritop® Bottle-top Filtration Units 0.22 μm (Millipore Express® PLUS)
MitoTrackerTM Deep Red 633 FM Dye (ThermoFischer Scientific, catalog number: M46753)
Mammalian Ringer (MR) (see Recipes)
MitoTrackerTM Deep Red 633 FM Dye stock solution (see Recipes)
Equipment
Pipetting Standard (Gilson, Pipetman®, models: P20, P200, and P1000) (http://www.gilson.com/Products/)
MicroCentrifuge (Eppendorf, model: 5417R)
Dissecting Scalpels and Blades, dissecting Scissors, dissecting dressing Forceps, as in United (ScientificTM Dissecting Set, catalog number: S111010)
Dewar flask
Red light/dark room
Vacuum filtration device
Procedure
A flowchart depicting the whole experimental procedure is shown in Figure 2.
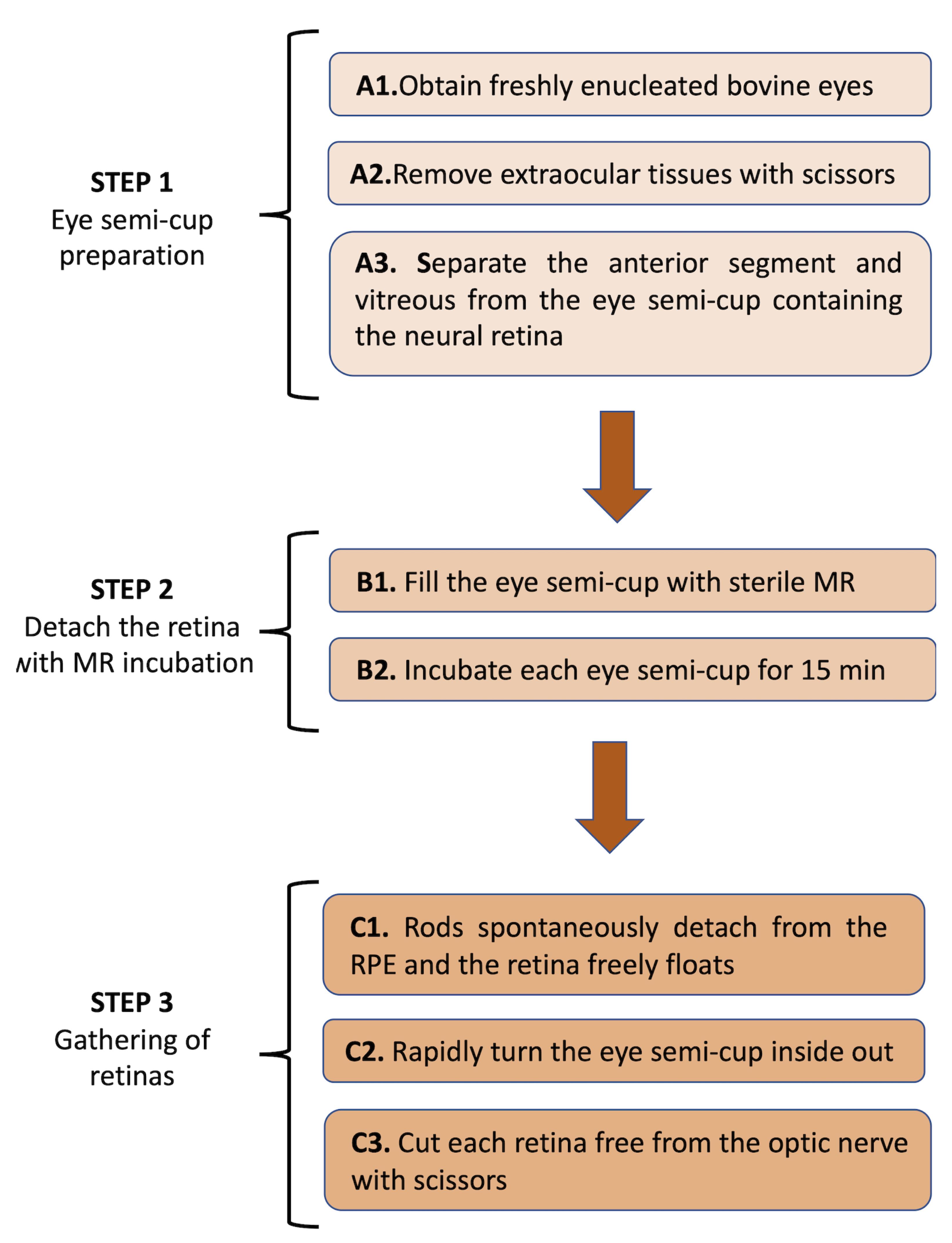
Figure 2. Flowchart of the procedure to detach the retina from the eye semi-cup with the MR incubation.
Prepare the eye semi-cup for incubation
Operations from step A2 on must be carried out at room temperature, in a dark room in dim red light (lower than 10 lux, 620 nm red lighting), to avoid rhodopsin bleaching. This facilitates the detachment of the photoreceptor OS from the RPE.
Obtain freshly enucleated bovine eyes from a slaughterhouse and use them within 2 h of animal death. The age of the cattle from which the eyes can be obtained for scientific purposes may be limited (as is the case in Italy) to 1.5 years, as a cautionary measure for the risk of possible transmission of Bovine Spongiform Encephalopathy (BSE). Eyes must be kept warm, in a light-tight container, possibly a Dewar flask, and handled to minimize the temperature decline.
Remove extraocular tissues with scissors.
Gently separate the anterior segment and vitreous from the eye semi-cup containing the neural retina. Make an incision in the sclera with a scalpel on one side of the eyeball, then hemi-sect the eye using scissors, approximately at the level of the ora serrata. Remove the anterior chamber, the lens, and vitreous carefully and discard them.
Detach the retina with MR incubation (Figure 3)
Fill the eye semi-cup containing the retina still attached to the RPE with 5–7 mL (depending on the size of the eye) of sterile filtered MR containing 2 mM glucose, protease inhibitor cocktail, and Ampicillin (100 μg/mL). All the operations, including incubations, are conducted at room temperature. The surface for dissection is routinely an aluminum foil.
Incubate each eye semi-cup for 10–15 min to allow the retina to detach completely. Eyes should be dissected and incubated with MR in rapid sequence.
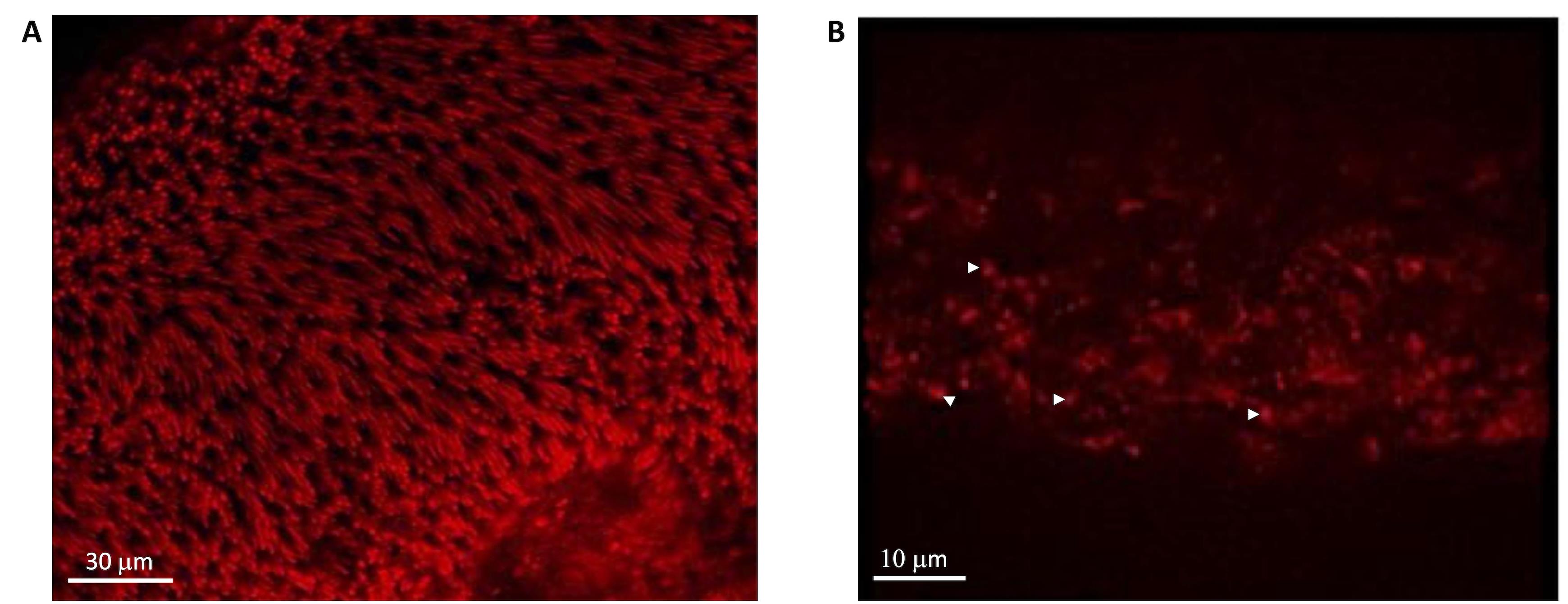
Figure 3. CLSM evaluation of OS distribution in the retina and the eye semi-cup after asportation with MR. A) Mitotracker signal in a whole retina spontaneously detached from the eye semi-cup. In this case, a homogeneous layer of photoreceptors is recognizable. B) Mitotracker signal in an eye semi-cup from which the retina was let float to detach spontaneously from RPE. In this case, only mitochondria are visible (white head arrows). MitoTracker Deep Red 633 was used, dissolved in DMSO, and kept at -20°C in dark vials.Gathering of Retinas (Figure 4)
After 10 min of incubation, rods spontaneously detach from the RPE, and the retina freely floats inside the eye semi-cup, attached to it through the optic nerve fibers; upon shorter incubation time, if it is necessary to remove the retina, gently shake the eye semi-cup by hand for 10 s, avoiding spilling the liquid contained inside, until the retina completely detaches from the RPE.
Rapidly turn the eye semi-cup inside out and discard the MR previously filling the semicup and now dripping out.
Cut each retina free from the optic nerve with scissors, letting the retina drop into a centrifuge 13 mL tube.
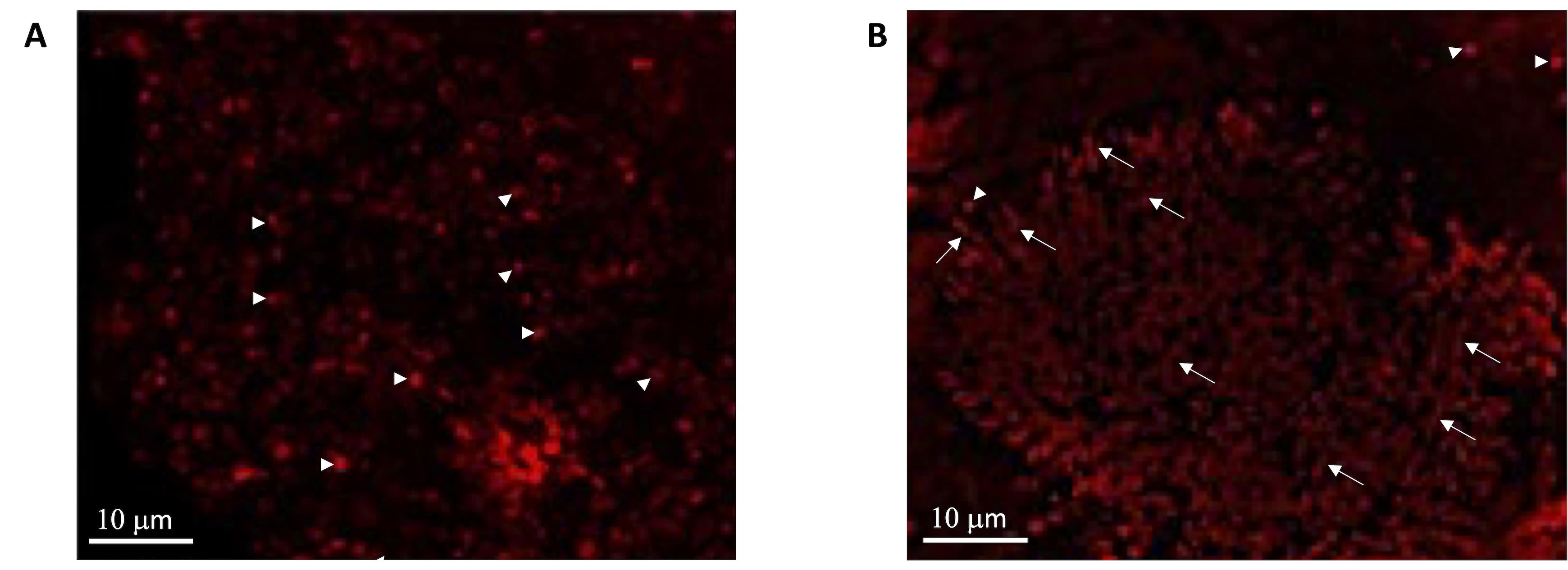
Figure 4. CLSM evaluation of OS distribution in the retina and the eye semi-cup after retina asportation with a soft brush. A) Retina detached from the eye semi-cup by a soft brush. In this case, the sample is characterized by a poor OS layer, allowing the visualization of the underlying mitochondria (white head arrow) belonging to inner segments of photoreceptors and the other retinas layers. B) Mitotracker signal in an eye semi-cup from which the retina had been detached with a soft brush. Specifically, it is possible to observe mitochondria (white head arrows) and OS immersed into the RPE (white arrows) since they have been torn from their ellipsoid.
Data analysis
The present method allows an increase of approximately 300% in the yield of total OS disk protein compared with the mechanical removal of the retinas from the eye semi-cup with a tweezer. When OS disks are purified by the method in Smith et al. (1975), the yield is 0.5 mg of rhodopsin/retina, corresponding to 0.58 mg protein/retina and approximately 11 mg total protein from 20 retinas (Smith et al., 1975). We have purified disks from 20 retinas utilizing the method described by Smith et al. (1975), extracting the retinas from the eye semi-cups, both following the present protocol and the mechanical method utilizing tweezers. The total disk protein yield, as assayed according to Bradford (Bradford, 1976), was 30 ± 2 mg of total disk protein in the first case and 10 ± 0.8 mg of total protein when retinas were extracted using tweezers.
Notes
In our experience of more than 10 years using this method, the reproducibility is excellent. Any eye of cattle up to 1.5 years of age (limitation due to BSE precaution rules) can be treated as described with predictable outcomes. On the other hand, if retinas are mechanically detached from the eye semi-cup, either with scissors or a brush, the yield in rod outer segment protein is extremely variable and can be approximately 300% lower. Additionally, our procedure maintains the physiological features of the retinal tissue.
Cautionary points:
(i) To prevent rhodopsin activation, avoid any white light source (McDowell and Kühn, 1977) by performing experiments in a dark/red light room;
(ii) Remove the vitreous humor from the eye semi-cup with special care, i.e., by gentle and rapid manual squeezing to avoid premature detachment of the retina, which would invalidate the present procedure;
(iii) Keep the eyes warm, minimizing the temperature drop, as eye cooling causes disaggregation of the RPE, which can contaminate the retinas, even when extracted with the present procedure;
(iv) As the bacterial dimension is similar to the rod disks (Smith and Litman, 1982), work in sterile conditions, filter sterilize the medium, use an antibiotic, and thoroughly clean the dissection tools with disinfectant soap and warm water.
Recipes
Mammalian Ringer (MR)
7.850 mL of 1 M NaCl
0.250 mL of 1 M KCl
1.750 mL of 200 mM Na2HPO4
2.000 mL of 200 mM NaH2PO4
0.025 mL of 1M MgCl2
Mix 0.025 of 1 M CaCl2 pH 6.9 in Milli-Q® water; add the protease inhibitor cocktail, according to the manufacturer instructions), and 100 μg/mL of Ampicillin.
When MT Deep Red 633 FM Dye is necessary, add it form the stock solution at 500 nM final concentration.
MitoTrackerTM Deep Red 633 FM Dye stock solution
Dissolve the mitochondrial dyes MT Deep Red in dimethylsulfoxide (DMSO) to make a 200 µM stock solutions and keep the solution at −20°C in dark vials.
Acknowledgments
This work was supported by FRA (Fondi per la Ricerca di Ateneo) 2019 from University of Genoa. This protocol was derived from previous original research articles, Calzia et al. (2018) and Ravera et al. (2020).
Competing interests
Authors declare absence of financial and non-financial competing interests.
Ethics
The present protocol utilizes bovine eyes from cattle intended for human consumption, which are killed at local slaughterhouses; therefore, ethic issues can be considered ruled out.
References
- Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248-254.
- Bruschi, M., Bartolucci, M., Petretto, A., Calzia, D., Caicci, F., Manni, L., Traverso, C. E., Candiano, G. and Panfoli, I. (2020). Differential expression of the five redox complexes in the retinal mitochondria or rod outer segment disks is consistent with their different functionality. FASEB Bioadv 2(5): 315-324.
- Bubis, J. (1998). Effect of detergents and lipids on transducin photoactivation by rhodopsin. Biol Res 31(1): 59-71.
- Calzia, D., Bianchini, P., Ravera, S., Bachi, A., Candiano, G., Diaspro, A. and Panfoli, I. (2010). Imaging of living mammalian retina ex vivo by confocal laser scanning microscopy. Anal Methods 2(11).
- Campbell, L. J., West, M. C. and Jensen, A. M. (2018). A high content, small molecule screen identifies candidate molecular pathways that regulate rod photoreceptor outer segment renewal. Sci Rep 8(1): 14017.
- de Grip, W. J., Daemen, F. J. and Bonting, S. L. (1972). Enrichment of rhodopsin in rod outer segment membrane preparations. Biochemical aspects of the visual process—XVIII. Vision Res 12(10): 1697-1707.
- Gilliam, J. C., Chang, J. T., Sandoval, I. M., Zhang, Y., Li, T., Pittler, S. J., Chiu, W. and Wensel, T. G. (2012). Three-dimensional architecture of the rod sensory cilium and its disruption in retinal neurodegeneration. Cell 151(5): 1029-1041.
- Masland, R. H. (2012). The neuronal organization of the retina. Neuron 76(2): 266-280.
- McDowell, J. H. and Kuhn, H. (1977). Light-induced phosphorylation of rhodopsin in cattle photoreceptor membranes: substrate activation and inactivation. Biochemistry 16(18): 4054-4060.
- Molday, R. S., Hicks, D. and Molday, L. (1987). Peripherin. A rim-specific membrane protein of rod outer segment discs. Invest Ophthalmol Vis Sci 28(1): 50-61.
- Molday, R. S. and Moritz, O. L. (2015). Photoreceptors at a glance. J Cell Sci 128(22): 4039-4045.
- Netter. (2018). Atlas of Human Anatomy. (Professional Edition). Including Student Consult Interactive Ancillaries and Guides. Elsevier.
- Palczewski, K. (2014). Chemistry and biology of the initial steps in vision: the Friedenwald lecture. Invest Ophthalmol Vis Sci 55(10): 6651-6672.
- Panfoli, I., Calzia, D., Ravera, S., Bianchini, P. and Diaspro, A. (2010). Immunochemical or fluorescent labeling of vesicular subcellular fractions for microscopy imaging. Microsc Res Tech 73(12): 1086-1090.
- Papermaster, D. S. (1982). Preparation of retinal rod outer segments. Methods Enzymol 81: 48-52.
- Raubach, R. A., Franklin, L. K. and Dratz, E. A. (1974). A rapid method for the purification of rod outer segment disk membranes. Vision Res 14(5): 335-337.
- Ravera, S., Calzia, D., Bianchini, P., Diaspro, A. and Panfoli, I. (2007). Confocal laser scanning microscopy of retinal rod outer segment intact disks: new labeling technique. J Biomed Opt 12(5): 050501.
- Smith, H. G., Jr. and Litman, B. J. (1982). Preparation of osmotically intact rod outer segment disks by Ficoll flotation. Methods Enzymol 81: 57-61.
- Smith, H. G., Jr., Stubbs, G. W. and Litman, B. J. (1975). The isolation and purification of osmotically intact discs from retinal rod outer segments. Exp Eye Res 20(3): 211-217.
- Stenkamp, D. L. (2015). Development of the Vertebrate Eye and Retina. Prog Mol Biol Transl Sci 134: 397-414.
- Uhl, R., Desel, H., Ryba, N. and Wagner, R. (1987). A simple and rapid procedure for the isolation of intact bovine rod outer segments (ROS). J Biochem Biophys Methods 14(3): 127-138.
- Young, R. W. (1967). The renewal of photoreceptor cell outer segments. J Cell Biol 33(1): 61-72.
- Zimmerman, W. F. and Godchaux, W., 3rd (1982). Preparation and characterization of sealed bovine rod cell outer segments. Methods Enzymol 81: 52-57.
Article Information
Publication history
Accepted: Mar 9, 2022
Published: Jul 20, 2022
Copyright
© 2022 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Panfoli, I., Calzia, D., Ravera, S., Bianchini, P. and Diaspro, A. (2024). Maximizing the Rod Outer Segment Yield in Retinas Extracted from Cattle Eyes. Bio-protocol 14(14): e4474. DOI: 10.21769/BioProtoc.4474.
Category
Neuroscience > Sensory and motor systems > Retina
Cell Biology > Tissue analysis > Tissue isolation
Cell Biology > Tissue analysis > Tissue imaging
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Tips for asking effective questions
+ Description
Write a detailed description. Include all information that will help others answer your question including experimental processes, conditions, and relevant images.
Share
Bluesky
X
Copy link