- Submit a Protocol
- Receive Our Alerts
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Measurement of Ascorbate Peroxidase Activity in Sorghum
Published: Vol 12, Iss 20, Oct 20, 2022 DOI: 10.21769/BioProtoc.4531 Views: 1457
Reviewed by: Khyati Hitesh ShahPrasad S. DalviAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Evaluating Plant Drought Resistance with a Raspberry Pi and Time-lapse Photography
Daniel N. Ginzburg and Seung Y. Rhee
Jan 20, 2023 1293 Views
Determination of Paraquat in Arabidopsis Tissues and Protoplasts by UHPLC-MS/MS
Mingming Zhao [...] Xiaochun Ge
Apr 5, 2023 424 Views

A Plate Growth Assay to Quantify Embryonic Root Development of Zea mays
Jason T. Roberts [...] David M. Braun
Oct 20, 2023 423 Views
Abstract
The ascorbate peroxidase (APX) is a widely distributed antioxidant enzyme. It differs from catalase and other peroxidases in that it scavenges/reduces reactive oxygen species (ROS) such as hydrogen peroxide (H2O2) to water using reduced ascorbate as the electron donor. It is advantageous over other similar antioxidant enzymes in scavenging ROS since ascorbate may react with superoxide, singlet oxygen, and hydroxyl radical, in addition to reacting with H2O2. The estimation of its activity is helpful to analyze the level of oxidative stress in living systems under stressful conditions. The present protocol was performed to analyze the impact of heavy metal chromium (Cr) toxicity on sorghum plants in the form of APX enzyme activity under the application of glycine betaine (GB) and arbuscular mycorrhizal fungi (AMF) as stress ameliorators. Plant defense strategies against heavy metals toxicity involve the utilization of APX and the instigation of AMF symbiotic system, as well as their possible collaboration with one another or with the plant antioxidant system; this has been examined and discussed in literature. In this protocol, an increased APX activity was observed on underlying functions and detoxification capabilities of GB and AMF that are typically used by plants to enhance tolerance to Cr toxicity.
Graphical abstract:
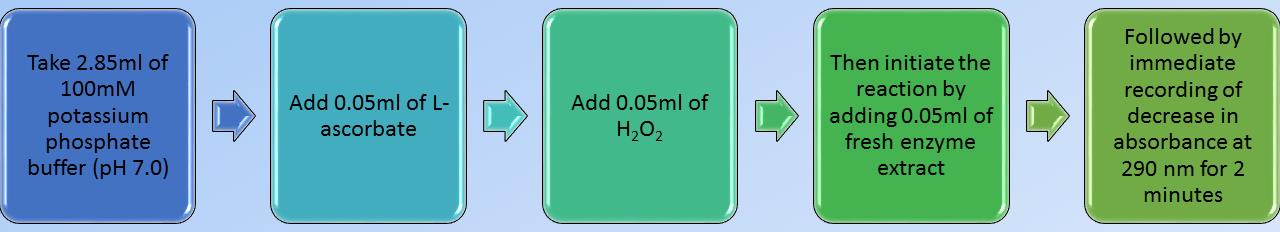
Flow chart of standardized or calibrated enzyme assay with leaf samples of sorghum
Background
Ascorbate peroxidase (APX) is an antioxidant enzyme, involved in the removal of toxic components such as reactive oxygen species (ROS) produced during physiologic and metabolic activities of the cell. Cells need to detoxify ROS efficiently as a consequence of abiotic stresses. A complex enzymatic antioxidative system could be developed by cells, which controls the production of ROS and ultimately protects the plant against oxidative damage (Ashraf et al., 2015). This metal-induced plant anti-oxidative defense mechanism is differential and largely based on the types of heavy metals and plant species. Elevated anti-oxidative enzyme activities attenuate abiotic oxidative stress and play a pivotal role in plants adapting against various environmental stresses (Singh et al., 2008). Furthermore, a decline in APX activity suggests that higher Cr toxicity might be inhibiting its ROS-eliminating role in plants. Decreased APX activity with increased metal toxicity was previously reported in Indian mustard (Mobin and Khan, 2007), oilseed rape (B. Ali, et al., 2014), and wheat (S. Ali, et al., 2015). Overall, increasing Cr toxicity triggers antioxidant production (mainly responsible for ROS quenching), representing plants’ potential to withstand Cr-polluted soils up to a certain level, which is further dependent on plant species and environmental conditions. Anjum et al. (2017) reported that at lower heavy metals concentrations the activity of antioxidant enzymes increased, whereas at higher concentrations the activity did not further increase. Samantaray et al. (1999) used peroxidase and catalase activities as enzyme markers for Cr-tolerant mung bean cultivars. The observed increase in antioxidant enzyme activity might have been in direct response to the generation of superoxide radicals by Cr-induced blockage of the electron transport chain in the mitochondria. Higher increases observed due to Cr(VI) indicated that its addition probably generated more singlet oxygen than Cr(III). The decrease in enzyme activity, as the concentration of external Cr increases, might be due to the inhibitory effect of Cr ions on the enzyme system itself.
In the present study, a comprehensive account of past developments and current trends using glycine betaine (GB) and arbuscular mycorrhizal fungi (AMF) in the research on Cr toxicity and its amelioration in sorghum plants has been attempted. Moreover, another line of plant defense strategy against heavy metals toxicity, which involves the utilization of APX and the instigation of AMF symbiotic system, as well as their possible collaboration with one another or with the plant antioxidant system, has been examined and discussed (Kumar, 2021). The study focuses on the underlying functions and detoxification capabilities of GB and AMF that are typically used by plants to enhance tolerance to Cr toxicity.
Materials and Reagents
Aluminum foil
Hydrogen peroxide, 35% in water (H2O2) (TCI, catalog number: H1222, CAS RN: 7722-84-1)
L(+)-ascorbic acid (CAS 50-81-7) (Merck Millipore, catalog number:100468)
0.1 M potassium phosphate buffer (pH 7.0) (see Recipes)
Reaction mixture (see Recipes)
Equipment
Scale
Pipettes
Stirrer
Mortar and pestle
Centrifuge machine
Double beam UV-VIS spectrophotometer with Graphic LCD – Typr 2205
Glassware: test tubes, beakers, conical flask, measuring cylinder, and a volumetric flask (Slisco scientific and Ace Glass Incorporated)
Procedure
Sample extraction
Note: The complete extraction procedure must be carried out at 0–4 °C. The ascorbate peroxidase activity was measured in leaf tissues of sorghum plants.
Completely homogenize 2 g of fresh and cleaned tissue sample in 10 mL of fresh 0.1 M potassium phosphate buffer (pH 7.0) by using a previously chilled mortar and pestle.
Centrifuge the homogenate at 11,180 × g for 15 min in a temperature-controlled centrifuge machine.
Collect the supernatant as crude extract and discard the pellet completely.
Use this enzyme extract immediately for enzyme assay and preserve the remaining extract in the refrigerator for total soluble protein estimation, which is required for enzyme specific activity calculation.
Enzyme assay
Note: APX is assayed by the method of Nakano and Asada (1981). For estimating APX activity use freshly prepared reagents and extracts only.
Add 3 mL of reaction mixture (see Recipes) and initiate the reaction by adding 50 µL of enzyme extract (from step A4) at the end.
The blank can be simultaneously prepared for each sample by taking 50 µL of boiled enzyme extract instead of enzyme extract.
Record the decrease in absorbance at 290 nm using the spectrophotometer for 2 min against a suitable blank.
The enzyme activity can be calculated using the molar extinction coefficient (absorbance of one molar solution) of 2.8 mM-1 cm-1 for ascorbate in the standard equation given below. Enzyme activity will be given in enzyme units, with one enzyme unit corresponding to the amount of enzyme required to oxidize 1 nmol of ascorbic acid per minute.
Standard equation for absorbance: A = ϵ × l × с
Where A is the amount of light absorbed by the sample at a given wavelength, ϵ is the molar extinction coefficient, l is the distance that the light travels through the solution, and с is the concentration of the absorbing species.
Note: To get better and more accurate results according to your samples and instruments, calibration between L-ascorbate, H2O2, and enzyme extract should be performed before starting the final procedure.
Calculations:
The enzyme activity and specific activity can be calculated as explained in Table 1 for a supposed sample size. The total protein content of the sample is required to calculate the specific activity, which can be determined by Lowry’s method.
Table 1. Ascorbate peroxidase activity (units or nmol of ascorbate/minute)
| Supposed sample used: extract was made from a 2 g sample of fresh leaves in 10 mL of potassium phosphate buffer; from this, 0.05 mL of extract was used for assay. Assume P mg/mL of protein was found per sample for the present case from Lowry’s method. | |||||||||||
| Absorbance at 290 nm | Enzyme activity and Specific activity | ||||||||||
| Replicate | Time 0 s | 15 s | 30 s | 45 s | 60 s | Decrease in absorbance | Average decrease in absorbance | Enzyme activity/minute (units) = activity/0.05 mL | Dilution factor (activity/mL of extract) | Total protein content (mg/mL) | Specific activity (µmol/min/mg) |
| 1st | a | b | c | d | e | a - e = F | (F + O + X) / 3 = Y | Y / 2.8 = Z | (Z / 0.05) × 1 = G | P | G / P = Q |
| 2nd | j | k | l | m | n | j - n = O | |||||
| 3rd | s | t | u | v | w | s - w = X | |||||
Recipes
0.1 M potassium phosphate buffer (pH 7.0)
0.5 L of 1 M K2HPO4 at 174.18 g mol-1 = 87.09 g
0.5 L of 1 M KH2PO4 at 136.09 g mol-1 = 68.045 g
Mix 61.5 mL of 1 M K2HPO4 with 38.5 mL 1 M KH2PO4 for the preparation of 0.1 M potassium phosphate buffer pH 7.0 at 25 °C
Reaction mixture
2.7 mL of 100 mM potassium phosphate buffer (pH 7.0)
0.1 mL of L(+)-ascorbic acid
0.15 mL of H2O2
Acknowledgments
This research was financially supported by CCS Haryana Agricultural University authority. The corresponding author deeply appreciates the CCSHA University and its staff members for their valuable assistance in experimental conductance and data analysis. My gratitude also goes to Dr. H. R. Singal for encouraging me to write this research article.
Competing interests
The authors declare that they have no conflicts of interest.
References
- Ali, B., Xu, X., Gill, R. A., Yang, S., Ali, S., Tahir, M. and Zhou, W. (2014). Promotive role of 5-aminolevulinic acid on mineral nutrients and antioxidative defense system under lead toxicity in Brassica napus. Ind Crops Prod 52: 617-626.
- Ali, S., Chaudhary, A., Rizwan, M., Anwar, H. T., Adrees, M., Farid, M., Irshad, M. K., Hayat, T. and Anjum, S. A. (2015). Alleviation of chromium toxicity by glycinebetaine is related to elevated antioxidant enzymes and suppressed chromium uptake and oxidative stress in wheat (Triticum aestivum L.). Environ Sci Pollut Res Int 22(14): 10669-10678.
- Anjum, S. A., Ashraf, U., Imran, K. H. A. N., Tanveer, M., Shahid, M., Shakoor, A. and Longchang, W. A. N. G. (2017). Phyto-toxicity of chromium in maize: oxidative damage, osmolyte accumulation, anti-oxidative defense and chromium uptake. Pedosphere 27(2): 262-273.
- Ashraf, U., Kanu, A. S., Mo, Z., Hussain, S., Anjum, S. A., Khan, I., Abbas, R. N. and Tang, X. (2015). Lead toxicity in rice: effects, mechanisms, and mitigation strategies--a mini review. Environ Sci Pollut Res Int 22(23): 18318-18332.
- Kumar, P. (2021). Soil applied glycine betaine with Arbuscular mycorrhizal fungi reduces chromium uptake and ameliorates chromium toxicity by suppressing the oxidative stress in three genetically different Sorghum (Sorghum bicolor L.) cultivars. BMC Plant Biology 21(1): 1-16.
- Mobin, M. and Khan, N. A. (2007). Photosynthetic activity, pigment composition and antioxidative response of two mustard (Brassica juncea) cultivars differing in photosynthetic capacity subjected to cadmium stress. J Plant Physiol 164(5): 601-610.
- Nakano, Y. and Asada, K. (1981). Hydrogen Peroxide is Scavenged by Ascorbate-specific Peroxidase in Spinach Chloroplasts. Plant Cell Physiol 22(5): 867-880.
- Samantaray, S., Rout, G. R. and Das, P. (1999). Studies on differential tolerance of mungbean cultivars to metalliferous minewastes. Agribiol Res 52(3-4):193-201.
- Singh, S., Khan, N. A., Nazar, R. and Anjum, N. A. (2008). Photosynthetic traits and activities of antioxidant enzymes in blackgram (Vigna mungo L. Hepper) under cadmium stress. Am J Plant Physiol 3(1): 25-32.
Article Information
Publication history
Accepted: Aug 21, 2022
Published: Oct 20, 2022
Copyright
© 2022 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Kumar, P. (2022). Measurement of Ascorbate Peroxidase Activity in Sorghum. Bio-protocol 12(20): e4531. DOI: 10.21769/BioProtoc.4531.
Category
Plant Science > Plant biochemistry > Metabolite
Plant Science > Plant physiology > Abiotic stress
Biochemistry > Other compound
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Tips for asking effective questions
+ Description
Write a detailed description. Include all information that will help others answer your question including experimental processes, conditions, and relevant images.
Share
Bluesky
X
Copy link







