- Submit a Protocol
- Receive Our Alerts
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
In vivo Drug Screening to Identify Anti-metastatic Drugs in Twist1a-ERT2 Transgenic Zebrafish
Published: Vol 13, Iss 10, May 20, 2023 DOI: 10.21769/BioProtoc.4673 Views: 358
Reviewed by: Xi FengAmr Galal Abdelraheem IbrahimAlberto Rissone

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

High-speed Atomic Force Microscopy Observation of Internal Structure Movements in Living Mycoplasma
Kohei Kobayashi [...] Makoto Miyata
Mar 5, 2022 2079 Views

Gastrulation Screening to Identify Anti-metastasis Drugs in Zebrafish Embryos
Joji Nakayama [...] Zhiyuan Gong
Oct 5, 2022 884 Views
An Unbiased CRISPR-Cas9 Screening Method for the Identification of Positive and Negative Regulatory Proteins of Cell Adhesion
Yvonne J. Thus [...] Marcel Spaargaren
Nov 5, 2022 900 Views
Abstract
Here, we present an in vivo drug screening protocol using a zebrafish model of metastasis for the identification of anti-metastatic drugs. A tamoxifen-controllable Twist1a-ERT2 transgenic zebrafish line was established to serve as a platform for the identification. By crossing Twist1a-ERT2 with xmrk (a homolog of hyperactive form of the epidermal growth factor receptor) transgenic zebrafish, which develop hepatocellular carcinoma, approximately 80% of the double transgenic zebrafish show spontaneous cell dissemination of mCherry-labeled hepatocytes from the liver to the entire abdomen and tail regions in five days, through induction of epithelial to mesenchymal transition (EMT). This rapid and high-frequency induction of cell dissemination makes it possible to perform an in vivo drug screen for the identification of anti-metastatic drugs targeting metastatic dissemination of cancer cells. The protocol evaluates the suppressor effect of a test drug on metastasis in five days, by comparing the frequencies of the fish showing abdominal and distant dissemination patterns in the test drug–treated group with those in the vehicle-treated group. Our study previously identified that adrenosterone, an inhibitor for hydroxysteroid (11-beta) dehydrogenase 1 (HSD11β1), has a suppressor effect on cell dissemination in the model. Furthermore, we validated that a pharmacologic and genetic inhibition of HSD11β1 suppressed metastatic dissemination of highly metastatic human cell lines in a zebrafish xenotransplantation model. Taken together, this protocol opens new routes for the identification of anti-metastatic drugs.
Graphical overview
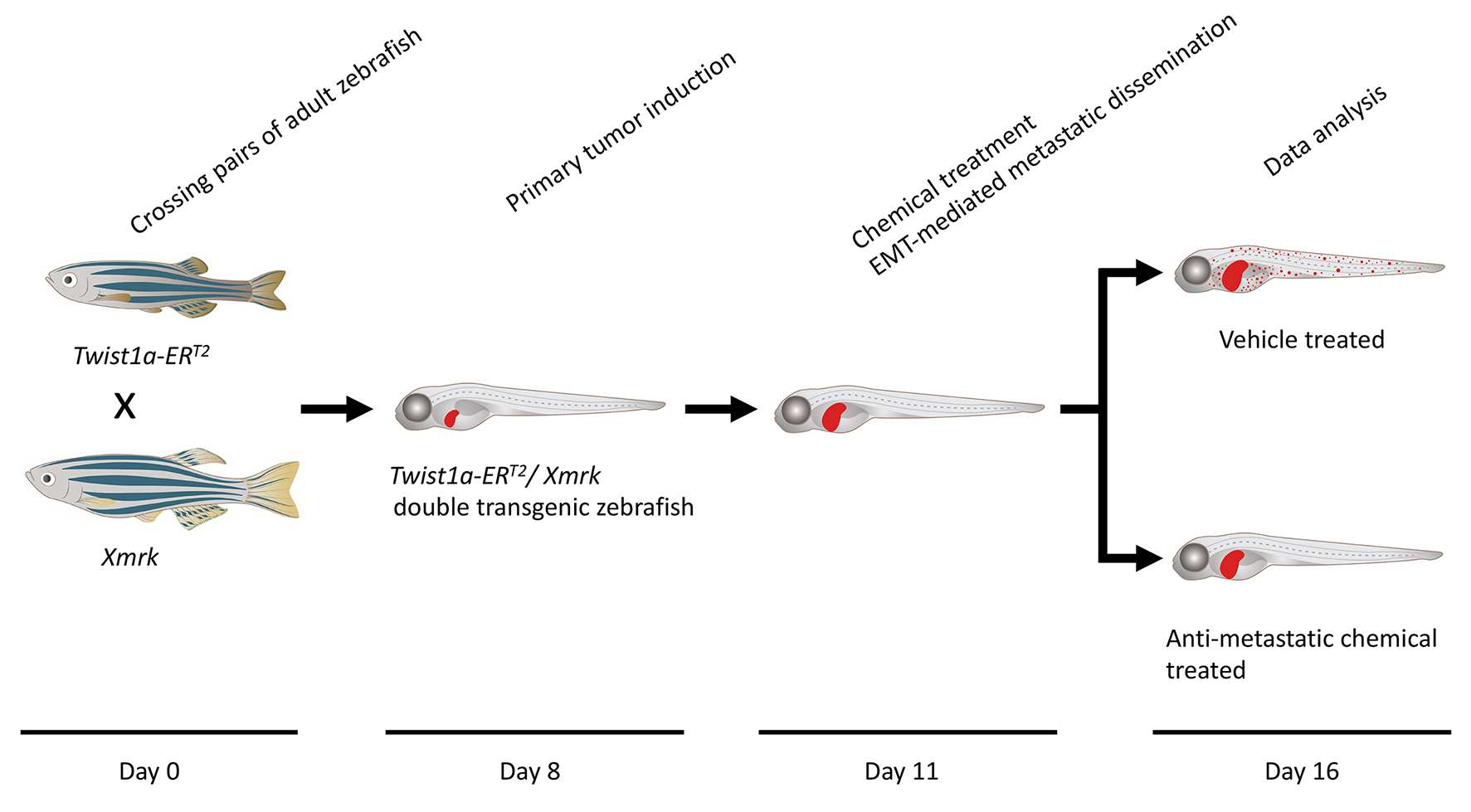
Timing
Day 0: Zebrafish spawning
Day 8: Primary tumor induction
Day 11: Chemical treatment
Day 11.5: Metastatic dissemination induction in the presence of a test chemical
Day 16: Data analysis
Key features
Keywords: In vivo drug screen
Graphical overview
Background
Metastasis is responsible for approximately 90% of cancer-associated mortality. It proceeds through multiple steps: invasion, intravasation, survival in the circulatory system, extravasation, colonization, and metastatic tumor formation in secondary organs with angiogenesis (Nguyen et al., 2009; Chaffer and Weinberg, 2011; Welch and Hurst, 2019). The dissemination of cancer cells is an initial step of metastasis, and its molecular mechanism involves a local breakdown of basement membrane, loss of cell polarity, and induction of epithelial to mesenchymal transition (EMT). EMT plays a central role in early embryonic morphogenesis; its process enables various types of epithelial cells to convert into mesenchymal cells, through a downregulation of epithelial markers such as E-cadherin and an upregulation of mesenchymal markers such as vimentin. Twist, a basic helix-loop-helix transcription factor, plays a critical role in inducing the EMT program (Tsai and Yang, 2013; Lu and Kang, 2019). Past studies showed that elevated expression of Twist is associated with poor survival rates in patients with cancer; also, ectopic expression of Twist confers metastatic properties on cancer cells through induction of EMT (Yang et al., 2004; Tsai et al., 2012).
Cancer research using zebrafish as a model has attracted attention because this model offers many unique advantages that are not readily provided by other animal models (White et al., 2013; Osmani and Goetz, 2019). Furthermore, the zebrafish system has also been increasingly recognized as a chemical screening platform because it provides the advantage of high-throughput screening in an in vivo vertebrate setting with physiologic relevance to humans (Zon and Peterson, 2005; Letrado et al., 2018; Nakayama and Gong, 2020; Nakayama and Makinoshima, 2020; Nakayama et al., 2021b, 2022a and 2022b). Our study previously established a tamoxifen-controllable Twist1a-ERT2 transgenic zebrafish line that serves as an in vivo drug screening platform for the identification of anti-metastasis drugs targeting metastatic dissemination of cancer cells. By crossing Twist1a-ERT2 with xmrk (a homolog of hyperactive form of the epidermal growth factor receptor) transgenic zebrafish, which develop hepatocellular carcinoma, approximately 80% of the double transgenic zebrafish showed spontaneous cell dissemination of mCherry-labeled hepatocytes from the liver to the entire abdomen and tail regions in five days, through induction of an EMT (Nakayama et al., 2020; Lu et al., 2021). The dissemination patterns are generally divided into three categories: (i) local dissemination, in which disseminated mCherry-positive cells exist in close proximity to the liver; (ii) abdominal dissemination, in which the cells spread throughout the abdomen; and (iii) distant dissemination, in which the cells are observed over a broad region from the trunk to the tail (Figure 1A).
This rapid and high-frequency induction of cell dissemination makes it possible to perform an in vivo drug screen for the discovery of anti-metastasis drugs targeting metastatic dissemination of cancer cells. The protocol evaluates the suppressor effect of a test chemical through comparing the frequencies of the fish showing the abdominal and distant dissemination patterns in the test drug–treated group with those in the vehicle-treated group. Previous studies confirmed that ki16425 (a LPA1 inhibitor) or Y27632 (an inhibitor of Rho-associated coiled-coil-containing protein kinase), which have been reported to suppress metastasis in mice models of metastasis (Itoh et al., 1999; Boucharaba et al., 2006), could suppress cell dissemination in the fish model. In vivo drug screen using this model identified adrenosterone, an inhibitor for hydroxysteroid (11-beta) dehydrogenase 1 (HSD11β1), as having a potential to suppress metastatic dissemination of cancer cells (Figure 1B and 1C). Furthermore, pharmacologic and genetic inhibition of HSD11β1 were validated to suppress metastatic dissemination of highly metastatic human cell lines in a zebrafish xenotransplantation model (Nakayama et al., 2020 and 2021a). Taken together, our model offers an in vivo drug screening platform for the identification of anti-metastatic drugs.
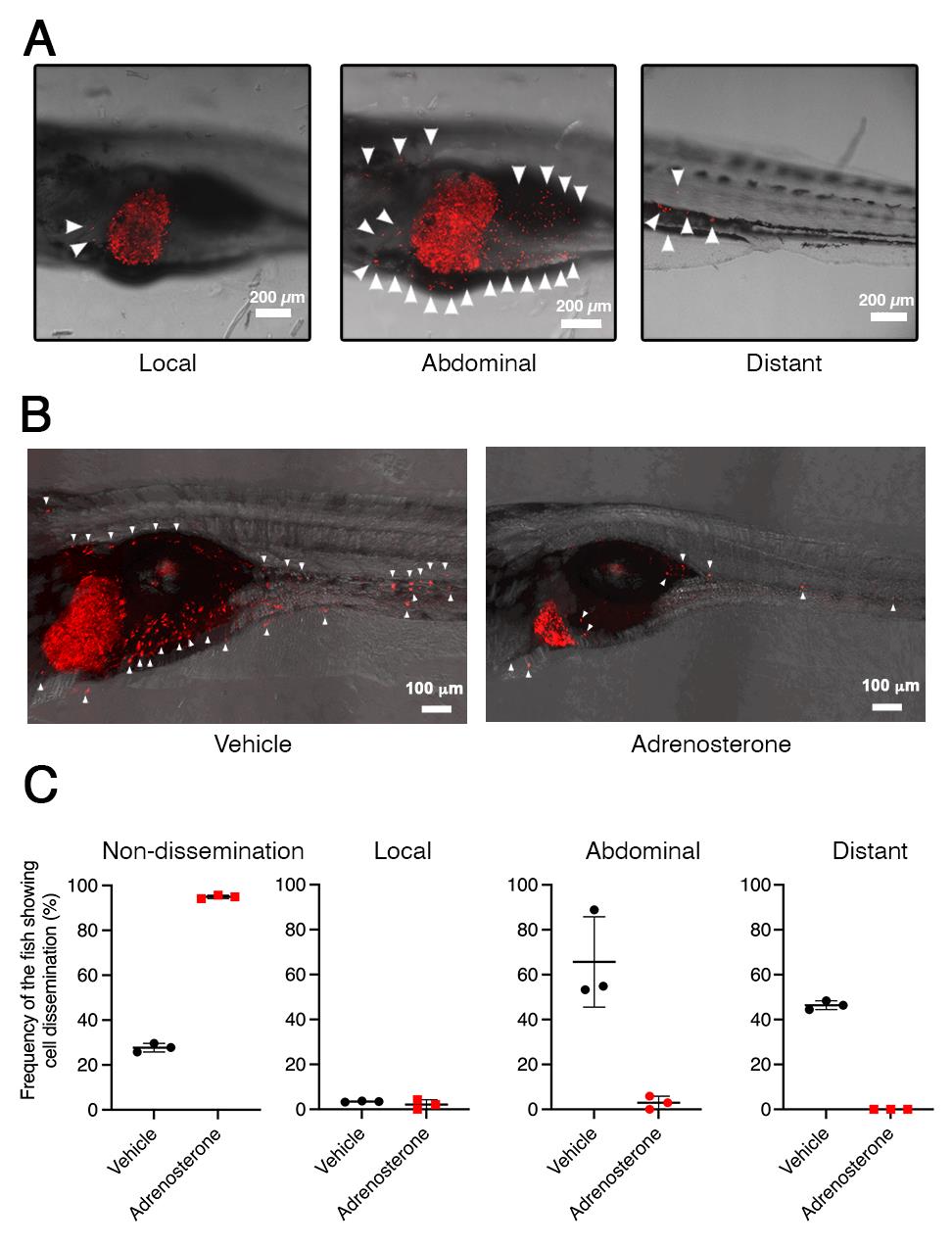
Figure 1. Twist1a-ERT2/xmrk double transgenic zebrafish offers an in vivo drug screening platform for the identification of anti-metastatic drugs. A. Representative images of the dissemination of mCherry-labeled hepatic cells from the liver in Twist1a-ERT2/xmrk double transgenic zebrafish at 16 days post-fertilization (dpf). The fish were treated with doxycycline and 4-hydroxytamoxifen (4-OHT). Some of the disseminated mCherry-positive cells are indicated by arrowheads. The images are shown as Z-stack images using 100× magnification. Scale bar, 200 μm. B. Representative images of the effect of adrenosterone on the dissemination of mCherry-positive cells in the fish at 16 dpf. Fish were treated with either vehicle (left) or adrenosterone (right). The images are shown as Z-stack images using 100× magnification. Scale bar, 100 μm. C. Mean frequencies of the fish showing the dissemination patterns of mCherry-positive cells in the vehicle- or adrenosterone-treated groups. Each value is presented as mean ± SEM from three independent experiments. Images are reprinted from Nakayama et al. (2020).
Materials and Reagents
150 mm dish (Corning, catalog number: 430599)
100 mm dish (Corning, catalog number: 430167)
6-well flat-bottom plastic plates (Corning, catalog number: CLS3335)
Micron powder food (food for zebrafish larvae) (Sera)
27 G needle tip (Terumo, catalog number: NN-2719S)
Plastic tea strainer (purchased from a local supermarket)
Transgenic zebrafish line Tg (fabp10a:mCherry-T2A-Twist1a-ERT2)
Transgenic zebrafish line Tg (fabp10a:TA; TRE:xmrk; krt4:GFP) known as xmrk
Note: Tg (fabp10a:mCherry-T2A-Twist1a-ERT2) and Tg (fabp10a:TA; TRE:xmrk; krt4:GFP) are available upon request from Prof. Zhiyuan Gong, Department of Biological Sciences, National University of Singapore, Singapore.
Doxycycline (Sigma-Aldrich, catalog number: D9891)
4-hydroxytamoxifen (4-OHT) (Sigma-Aldrich, catalog number: H6278)
Methylcellulose (Sigma-Aldrich, catalog number: M7027)
Phenoxyethanol (Sigma-Aldrich, catalog number: 77699)
NaCl (Sigma-Aldrich, catalog number: S3014)
KCl (Sigma-Aldrich, catalog number: P9541)
MgSO4·7H2O (Sigma-Aldrich, catalog number: M2773)
CaCl2 (Sigma-Aldrich, catalog number: C4901)
Doxycycline stock solution (see Recipes)
4-hydroxytamoxifen stock solution (see Recipes)
E3 medium (see Recipes)
30% methylcellulose (w/v %) (see Recipes)
Phenoxyethanol (v/v %) (see Recipes)
Equipment
Recirculating aquaculture system (Aquatic Habitat)
Fluorescence microscope (Olympus, catalog number: MVX10)
Leica TCS SP5X confocal microscope system (Leica)
Incubator for zebrafish embryos (AQUALYTIC, catalog number: 2418210)
External tank, a component of zebrafish breeding tank (Tecniplast, catalog number: ZB10BTE)
Perforated internal tank, a component of zebrafish breeding tank (Tecniplast, catalog number: ZB10BTI)
Polycarbonate divider, a component of zebrafish breeding tank (Tecniplast, catalog number: ZB10BTD)
Polycarbonate lid (Tecniplast, catalog number: ZB10BTL)
Software
Image analysis software, Imaris 8 (Bitplane)
Procedure
Zebrafish spawning (Day 0)
On the night before collecting the embryos, arrange pairs of male xmrk fish and female Twist1a-ERT2 fish in a zebrafish breeding tank with a divider.
Early in the morning, remove the divider to allow the fish to spawn.
Scoop out the embryos with a plastic tea strainer.
Transfer the embryos into a 150 mm plastic dish containing E3 medium.
Remove dead embryos with a pipette.
Maintain the embryos for six days in an incubator set at 27 °C.
Change the E3 medium and remove dead embryos with a pipette daily.
Notes:
Adult Twist1a-ERT2 and xmrk transgenic zebrafish lines were maintained in a recirculating aquaculture system with an ambient water temperature of approximately 28 °C in Singapore. 6–8 pairs of female heterozygous Twist1a-ERT2 transgenic fish and male heterozygous xmrk transgenic fish were crossed in the breeding tank (one pair per tank). Young adult zebrafish (3–9 months of age) were preferentially used for the crossing. Electric lighting in the facility was switched on from 07:00 to 19:00. Brine shrimps were fed to both zebrafish lines five times a day, at 08:30, 10:30, 12:30, 14:30, and 16:30.
The Fish facility at the National University of Singapore connects to the outdoors. Therefore, the facility does not have air conditioning and the room temperature in the facility fluctuates between 25 °C and 30 °C. Embryos and larvae are maintained in an incubator set at 27 °C. Therefore, all larvae zebrafish were maintained at 27 °C to ensure consistency of the zebrafish development.
If a specific developmental stage is required, the embryos should be grown at the optimal condition of 28.5 °C, in order to allow a standard development of the embryos and larvae (Westerfield, 2007).
Feeding of the zebrafish larvae began at 5 dpf. A pinch of micron powder was diluted in 1 mL of E3 medium and added to the zebrafish larvae every morning. The zebrafish larvae were transferred into each well of a 6-well plate at a time point between 8 and 11 dpf, and a few drops of the E3 medium were added to each well every morning.
Collect Twist1a-ERT2/xmrk double transgenic zebrafish (Day 6)
Transfer the zebrafish larvae into a 50 mL tube.
To anesthetize the fish, add 2% (v/v) phenoxyethanol to the E3 medium with the micron powder that contains the fish. Thus, the final concentration of phenoxyethanol is 0.02%.
Array the fish on a lid of a 150 mm plastic dish.
Visualize under a fluorescence microscope and use a pipette to collect the fish expressing green fluorescent protein (GFP) in the skin (Figure 2).
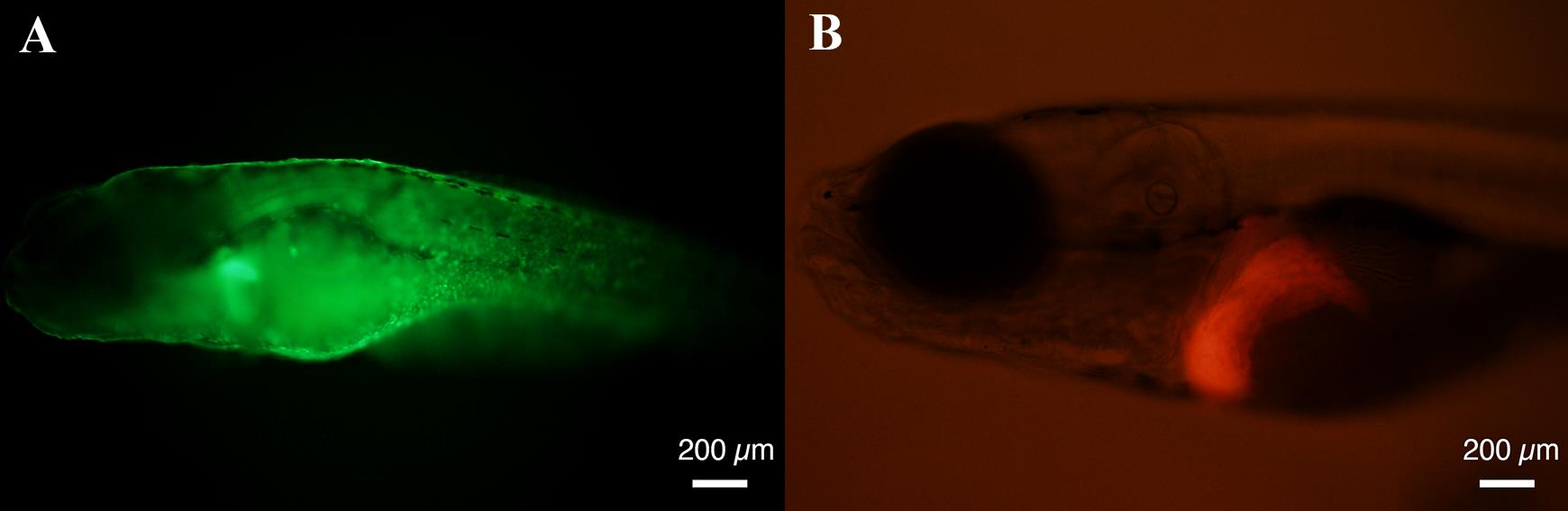
Figure 2. A Twist1a-ERT2/xmrk double transgenic zebrafish expresses green fluorescent protein (GFP) in a skin-specific manner (A) and mCherry in a liver-specific manner (B). Representative images of GFP and mCherry signals in Twist1a-ERT2/xmrk double transgenic zebrafish at 6 days post-fertilization (dpf). Scale bar, 200 μm.Among the GFP-positive fish, visualize under a fluorescence microscope and use a pipette to collect those expressing mCherry in the liver (Figure 2).
Transfer the collected fish into a 150 mm plastic dish containing E3 medium.
Maintain the fish for two days in an incubator set at 27 °C.
Notes:
At 6 dpf, Tg (fabp10a:mCherry-T2A-Twist1a-ERT2) begins to express the gene coding mCherry-T2A-Twist1a-ERT2 in a liver-specific manner. Tg (fabp10a:TA; TRE:xmrk; krt4:GFP) expresses GFP in a skin-specific manner. Therefore, Twist1a-ERT2/xmrk double transgenic zebrafish is indicated as mCherry- and GFP-positive in the liver and skin, respectively.
The screening process is divided into two steps. Firstly, zebrafish possessing the xmrk transgene are screened at 3–5 dpf and then the xmrk transgenic zebrafish possessing the Twist1a-ERT2 transgene are screened at 6 dpf.
The zebrafish larvae should not be overcrowded in the 150 mm plate, since overcrowding negatively affects their viability. By 6 dpf, 100–300 zebrafish larvae can be maintained in the 150 mm plate. After 6 dpf, a maximum of 100 zebrafish larvae should be maintained in the 150 mm plate.
Primary tumor induction (Day 8)
Aliquot a maximum of 100 zebrafish larvae into a 150 mm plate along with 100 mL of E3 medium.
At 8 dpf, treat the fish with 30 μg/mL of doxycycline in E3 medium for three days.
Change the E3 medium containing doxycycline every two days.
Note: 100 μL of doxycycline stock solution (30 mg/mL) is added to 100 mL of E3 medium.
Chemical treatment (Day 11)
Aliquot approximately 20 zebrafish larvae into each well of a 6-well plate with 8 mL of E3 medium containing doxycycline (30 μg/mL) at a time point between 8 and 11 dpf.
Add each test chemical to each well of the plate at a final concentration of 5 μmol/L.
Notes:
All test chemicals are dissolved in DMSO at a concentration of 10 mmol/L (stock solution).
4 μL of each stock solution (10 mmol/L) is diluted with 100 μL of E3 medium and added into each well of the plate containing 8 mL of E3 medium.
Metastatic dissemination induction in the presence of a chemical (Day 11.5)
Beginning 12 h after the addition of the test chemical, treat the fish with 0.1 μmol/L of 4-OHT in E3 medium for five days.
Change the E3 medium containing doxycycline, 4-OHT, and the test chemical every two days.
Notes:
The quality of the E3 medium is critical for the survival of the zebrafish larvae. The fish excrete waste materials including urine. Waste materials and leftover foods are harmful for the viability of the fish. Thus, the E3 medium should be changed before a high concentration of waste materials is reached.
We recommend changing the E3 medium every two days after 8 dpf.
Data analysis
Timing: Day 16
At five days after the first 4-OHT addition, transfer the zebrafish larvae into a 50 mL tube.
To anesthetize the fish, add 160 μL of 2% phenoxyethanol (v/v) into E3 medium containing the fish. The final concentration of phenoxyethanol is 0.02% (v/v) in a total volume of 16 mL.
Array the fish on the lid of a 100 mm plastic dish.
Embed the fish with a drop of 30% methylcellulose.
Manually orient the fish into a lateral view using a 27 G needle tip.
Determine the pattern of cell dissemination in the fish under a fluorescence microscope.
Count the number of fish showing each dissemination pattern of mCherry-labeled cells from the liver.
For taking images, capture serial sections of the fish in 8 μm Z-step intervals using a Leica TCS SP5X confocal microscope system. Process Z-stack images using the image analysis software Imaris (Bitplane).
Notes:
The dissemination patterns are generally divided into three categories: (i) local dissemination, in which disseminated mCherry-positive cells exist in close proximity to the liver; (ii) abdominal dissemination, in which the cells spread throughout the abdomen; and (iii) distant dissemination, in which the cells are observed over a broad region from the trunk to the tail (Figure 1).
The suppressor effect of a test drug is evaluated by comparing the frequencies of the fish showing the abdominal and distant dissemination patterns in the test drug–treated group with those in the vehicle-treated group.
The dissemination patterns are classified by viewing the fish under a fluorescence microscope. To avoid bias, the experimenter should be blinded to the treatments.
Limitations
There is a limitation on the number of Twist1a-ERT2/xmrk double transgenic zebrafish that can be prepared (a few hundred). This limitation determines how many chemicals can be tested in one screening session. To test one chemical, approximately 20 double transgenic fish are needed. Following Mendel's laws, the rate of the double transgenic fish production is 25% when a heterozygous Twist1a-ERT2 transgenic zebrafish is crossed with a heterozygous xmrk transgenic zebrafish. If 20 chemicals are to be tested, at least 400 double transgenic fish would be required. To prepare 400 double transgenic fish, pairs of Twist1a-ERT2 and xmrk fish would need to generate approximately 2,000 embryos. Thus, when following the protocol described above, a maximum of 20 test chemicals can be evaluated.
Recipes
Doxycycline stock solution
Reagent Final concentration Amount Doxycycline 50 mg/mL 500 mg ddH2O n/a 10 mL Total n/a 10 mL 4-hydroxytamoxifen stock solution
Reagent Final concentration Amount 4-OHT 100 mM 387 mg Ethanol n/a 1,000 mL Total n/a 1,000 mL E3 medium
Reagent Final concentration Amount NaCl 5.0 mM 0.292 g KCl 0.17 mM 0.013 g MgSO4·7H2O
CaCl2
ddH2O
Total
0.33 mM
0.33 mM
n/a
0.081 g
0.048 g
1,000 mL
1,000 mL
30% methylcellulose (w/v %)
Reagent Final concentration Amount Methylcellulose 30% 30 g ddH2O n/a 100 mL Total n/a 100 mL Phenoxyethanol (v/v %)
Reagent Final concentration Amount Phenoxyethanol 0.02% 20 μL ddH2O n/a 100 mL Total n/a 100 mL
Acknowledgments
Graphical abstract was drawn by Ami Inoue (Kyoto University of the Arts). This study was funded by Ministry of Education of Singapore (MOE2019-T2-2-018) to Z. Gong. This protocol originates from Nakayama et al. (2020).
Competing interests
J.N., H.M., and Z.G. declare no conflict of interest.
Ethics
The study protocol was approved by the Institutional Animal Care and Use Committee of the National University of Singapore (protocol number: 096/12).
References
- Boucharaba, A., Serre, C.-M., Guglielmi, J., Bordet, J.-C., Clézardin, P. and Peyruchaud, O. (2006). The type 1 lysophosphatidic acid receptor is a target for therapy in bone metastases. Proc Natl Acad Sci U S A 103(25): 9643-9648.
- Chaffer, C. L. and Weinberg, R. A. (2011). A perspective on cancer cell metastasis. Science 331(6024): 1559-1564.
- Itoh, K., Yoshioka, K., Akedo, H., Uehata, M., Ishizaki, T. and Narumiya, S. (1999). An essential part for Rho-associated kinase in the transcellular invasion of tumor cells. Nat Med 5(2): 221-225.
- Letrado, P., de Miguel, I., Lamberto, I., Díez-Martínez, R. and Oyarzabal, J. (2018). Zebrafish: Speeding Up the Cancer Drug Discovery Process. Cancer Res 78(21): 6048-6058.
- Lu, J.-W., Sun, Y., Lin, L.-I., Liu, D. and Gong, Z. (2021). Exacerbation of Liver Tumor Metastasis in twist1a+/ xmrk+ Double Transgenic Zebrafish following Lipopolysaccharide or Dextran Sulphate Sodium Exposure. Pharmaceuticals (Basel) 14(9): 867.
- Lu, W. and Kang, Y. (2019). Epithelial-Mesenchymal Plasticit in Cancer Progression and Metastasis. Dev Cell 49(3): 361-374.
- Westerfield, M. (2007). The Zebrafish Book, 5th Edition. A guide for the laboratory use of zebrafish (Danio rerio), Eugene, University of Oregon Press. Kimmel, C. B. et al. (1995). Stages of embryonic development of the zebrafish. Dev Dyn 203(3): 253-310.
- Nakayama, J. and Gong, Z. (2020). Transgenic zebrafish for modeling hepatocellular carcinoma. MedComm 1(2): 140-156.
- Nakayama, J., Konno, Y., Maruyama, A., Tomita, M. and Makinoshima, H. (2022a). Cinnamon bark extract suppresses metastatic dissemination of cancer cells through inhibition of glycolytic metabolism. J Nat Med 76(3): 686-692.
- Nakayama, J. and Makinoshima, H. (2020). Zebrafish-Based Screening Models for the Identification of Anti-Metastatic Drugs. Molecules 25(10): 2407.
- Nakayama, J., Makinoshima, H. and Gong, Z. (2022b). Gastrulation Screening to Identify Anti-metastasis Drugs in Zebrafish Embryos. Bio Protoc 12(19): e4525.
- Nakayama, J., Maruyama, A., Ishikawa, T., Nishimura, T., Yamanaka, S., Gotoh, N., Yamauchi, C., Onishi, T., Soga, T., Fujii, S., et al. (2021a). HSD11β1 promotes EMT-mediated breast cancer metastasis. bioRxiv doi: https://doi.org/10.1101/2021.09.27.461934
- Nakayama, J., Tan, L., Li, Y., Goh, B. C., Wang, S., Makinoshima, H. and Gong, Z. (2021b). A zebrafish embryo screen utilizing gastrulation identifies the HTR2C inhibitor pizotifen as a suppressor of EMT-mediated metastasis. Elife 10: e70151.
- Nakayama., J., Lu., J.-W., Makinoshima., H. and Gong., Z. (2020). A Novel Zebrafish Model of Metastasis Identifies the HSD11β1 Inhibitor Adrenosterone as a Suppressor of Epithelial-Mesenchymal Transition and Metastatic Dissemination. Mol Cancer Res 18(3): 477-487.
- Nguyen., D. X., Bos., P. D. and Massagué., J. (2009). Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer 9(4): 274-284.
- Osmani, N. and Goetz, J. G. (2019). Multiscale Imaging of Metastasis in Zebrafish. Trends Cancer 5(12): 766-778.
- White, R., Rose, K. and Zon, L. (2013). Zebrafish cancer: the state of the art and the path forward. Nat Rev Cancer 13(9): 624-636.
- Tsai, J. H., Donaher, J. L., Murphy, D. A., Chau, S. and Yang, J. (2012). Spatiotemporal regulation of epithelial-mesenchymal transition is essential for squamous cell carcinoma metastasis. Cancer Cell 22(6): 725-736.
- Tsai, J. H. and Yang, J. (2013). Epithelial–mesenchymal plasticity in carcinoma metastasis. Genes Dev 27(20): 2192-2206.
- Welch, D. R. and Hurst, D. R. (2019). Defining the Hallmarks of Metastasis. Cancer Res 79(12): 3011-3027.
- Yang, J., Mani, S. A., Donaher, J. L., Ramaswamy, S., Itzykson, R. A., Come, C., Savagner, P., Gitelman, I., Richardson, A. and Weinberg, R. A. (2004). Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell 117(7): 927-939.
- Zon, L. I. and Peterson, R. T. (2005). In vivo drug discovery in the zebrafish. Nat Rev Drug Discov 4(1): 35-44.
Article Information
Publication history
Published: May 20, 2023
Copyright
© 2023 The Author(s); This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/).
How to cite
Nakayama, J., Makinoshima, H. and Gong, Z. (2023). In vivo Drug Screening to Identify Anti-metastatic Drugs in Twist1a-ERT2 Transgenic Zebrafish. Bio-protocol 13(10): e4673. DOI: 10.21769/BioProtoc.4673.
Category
Drug Discovery > Drug Screening
Cancer Biology > Invasion & metastasis > Drug discovery and analysis
Cell Biology > Cell movement > Cell motility
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Tips for asking effective questions
+ Description
Write a detailed description. Include all information that will help others answer your question including experimental processes, conditions, and relevant images.
Share
Bluesky
X
Copy link







