- Submit a Protocol
- Receive Our Alerts
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Agrobacterium-mediated Genetic Transformation of Cotton and Regeneration via Somatic Embryogenesis
Published: Vol 13, Iss 10, May 20, 2023 DOI: 10.21769/BioProtoc.4677 Views: 1115
Reviewed by: Amey RedkarAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Agrobacterium-mediated Transformation of Japonica Rice Using Mature Embryos and Regenerated Transgenic Plants
Ammar Elakhdar [...] Takahiko Kubo
Sep 20, 2021 3602 Views

Rhizoctonia solani Infection Assay of Young Sugar Beet and Arabidopsis plantlets
Fredrik Dölfors [...] Christina Dixelius
Jan 20, 2022 2351 Views
Botrytis cinerea in vivo Inoculation Assays for Early-, Middle- and Late-stage Strawberries
Piao Yang [...] Ye Xia
Oct 20, 2023 292 Views
Abstract
Cotton is a significant industrial crop, playing an essential role in the global economy that suffers several setbacks due to biotic and abiotic adversities. Despite such problems, biotechnological advances in cotton are limited because of genetic transformation and regeneration limitations. Here, we present a detailed protocol optimized based on previously published papers, along with our modifications. These involve changes in Agrobacterium concentration, co-cultivation time and temperature, hormones used for regeneration, media manipulation for embryogenic callus production, and efficient rescue of deformed embryos. Further, this protocol has been used in genetic studies on biotic and abiotic stress in cotton. This protocol assures a reproducible stable transgenic cotton development procedure via somatic embryogenesis that can be used by researchers worldwide.
Keywords: CottonBackground
Genetic engineering has opened the doors to enhanced yield, disease resistance, and improved nutritional quality and quantity. Genetically modified (GM) crops ensure that the needs of the growing population are met worldwide. The altered attributes in GM crops are due to introduced transgenes, which is achieved by different tools as particle bombardment, chloroplast transformation, pollen tube pathway transformation, protoplast electroporation, Agrobacterium-mediated genetic transformation, and many more. Amongst these, Agrobacterium tumefaciens–mediated transformation is the preferred one due to its exceptional trait of transferring a DNA segment into the host cell via a specialized plasmid (Ziemienowicz, 2014). This technique has become favorable for scientists and industries because of its simple and easy approach, its ability to transfer a low copy number of the gene of interest, and its efficiency of transfer of a large DNA fragment into the host cell—all of this at a very low cost. Agrobacterium-mediated genetic transformation has been employed to transform many economically important crops using plant tissue culture, which helps to regenerate a large number of transgenic plants at a single time point (Somleva et al., 2002; Manickavasagam et al., 2004; Jones et al., 2005; Sun et al., 2006).
Transgenic cotton was one of the first genetically modified organisms to be globally accepted and commercially developed. Now and again, researchers have been trying to improve the quality of cotton crops, either by enhancing its fiber yield or by imparting pests resistance. Developing a transgenic cotton plant is tedious and time consuming because of its recalcitrant nature and high production of phenolics, which hinders the regeneration of the plant. Although Agrobacterium-mediated genetic transformation has enabled the development of stable transgenic cotton, various efforts have been made to improve the transformation process (Qandeel-E-Arsh et al., 2021). A successful transformation does not depend on a single parameter; instead, it heavily relies on multiple factors such as Agrobacterium strain, type and age of explants, duration of co-cultivation between Agrobacterium and the plant tissue, bacterial cell density during transformation, regeneration method for the plant, selection marker, and type of vector to use (Firoozabady et al., 1987; Satyavathi et al., 2002; Jadhav and Katageri, 2017). To develop transgenic cotton plants, scientists have used the hypocotyls, cotyledons, and shoot tips, as well as the embryogenic callus, pollen tube, embryo, and meristems as explants (Jin et al., 2005; Pathi and Tuteja, 2013; Wang et al., 2013). The use of meristems is very cumbersome and labor intensive and requires particle bombardment (with the drawback of high copy number). The use of the embryonic callus is a relatively good option, but these take several months to be obtained, and the subsequent process does not guarantee proper plantlet germination (McCabe and Martinell, 1993). Transformation of the shoot tip, the embryo, and the pollen tube is easier than others, but the potential for stable transgenic plants is low. Most of these techniques fall into the category of transient transformation, skipping the inserted gene in subsequent generations. Direct organogenesis has also been tried in order to facilitate regeneration, but when the transformation is involved, it becomes sluggish and dreary, and chimeric plants are often obtained. Many of these issues can be resolved by using hypocotyls or cotyledons as the explant, as the process involves somatic embryogenesis that produces somatic embryos, which are single cell–derived. Furthermore, this promotes high-end vegetative output, ensuring more transgenic plants.
Various regeneration protocols have been proposed by several researchers. They have fiddled with different concentrations and types of plant hormones to induce callus, manipulated growth media to induce embryogenesis in proliferating calli, and changed carbohydrate sources as well (Shoemaker et al., 1986; Finer, 1988; Hemphill et al., 1998; Aydin et al., 2004; Ikram-ul-Haq and Zafar, 2004; Kumar and Tuli, 2004). All these attempts have successfully regenerated cotton genotypes, but the development of stable transgenic lines requires an appropriate combination of cotton regeneration and transformation. Currently, the most commonly used approaches are the pollen tube pathway, embryo transformation, and meristem-mediated methods (Bajwa et al., 2015), but these do not guarantee stable gene transfer. Using these methods, elucidation of the applied hypothesis is possible, but only a few have been able to be applied in practical use for stable transgenic development.
Here, we propose a protocol for Agrobacterium-mediated genetic transformation and regeneration in cotton, resulting in transgenic production (Figure 1). This protocol is based on the approach described by Kumar and Tuli (2004), with various modifications that accelerate the achievement of the transgenic development. We have optimized the media for the Agrobacterium to be ready to infect the explants, the co-cultivation period, the appropriate quantity and type of phytohormones to be used, and the environment required for callus induction, its proliferation, and further embryogenesis, and we have reduced the abnormalities in the embryos produced. To summarize, this protocol is an approach towards obtaining a stable transgenic line, via transforming the explants with a gene of interest and regenerating them with early callus induction, improved embryogenesis, and culture requisites. We have used a 14 kb construct harboring a CamV35S promoter, the pectin methylesterase (PME) gene, the neomycin phosphotransferase II (nptII), and the nopaline synthase (Nos) terminator. The protocol has been used to develop several distinct transgenic lines and is currently being used to alter numerous unique genes in cotton for genome editing.
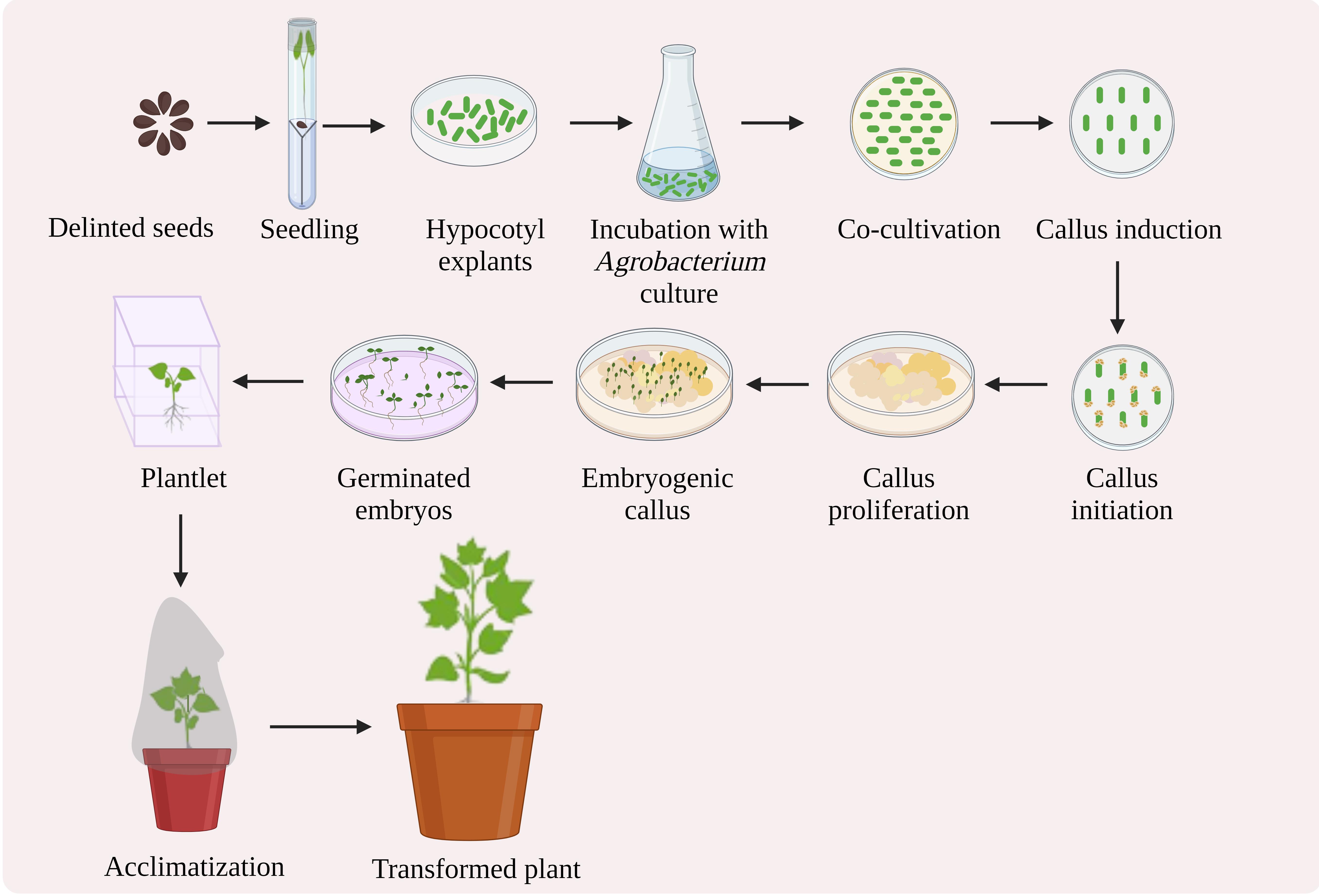
Figure 1. Schematic representation of Agrobacterium-mediated genetic transformation of cotton hypocotyl explants and regeneration (created with BioRender.com)
Materials and Reagents
Glassware
Glass rod
Conical flask, 250 mL (Borosil, catalog number: 4984021)
Glass bottle, 1,000 mL (Borosil, catalog number: 1501029)
Culture vials, 30 mL (Borosil, catalog number: 9910010)
Test tubes
Wide-mouth bottles, 100 mL
Plasticwares
Petri plates, 90 × 20 mm (Tarsons, catalog number: 960096)
Petri plates, 90 × 15 mm (Tarsons, catalog number: 960091)
Petri plates, 100 × 20 mm (Genetix, catalog number: 310100)
Parafilm tape (Tarsons, catalog number: 380020)
Stainless steel sieve
Filter paper (Whatman, catalog number: 1003-917)
Computer paper
Planton plant tissue culture container, 7.5 × 7.5 × 10 cm (Tarsons, catalog number: 020080)
Frames for planton boxes (Genetix, catalog number: 310074)
Spirit lamp
Plastic bags
Falcon tubes, 50 mL
Sterile syringe
Test tube caps (Tarsons, catalog number: 020070)
Plant earthen pots, 14”
Plant plastic pots, 5”
Sterile syringe filters, 0.22 μm
Electroporation cuvettes (gene pulser 0.4 cm gap) (Bio-Rad, catalog number: 1652086)
Toothpick
Scalpel
Forceps
Surgical blade
Biological materials
Matured, defuzzed, and delinted cotton seeds. Variety: Coker-312
Agrobacterium tumefaciens strain LBA4404
Binary vector incorporating gene of interest. Here, PME (pectin methylesterase gene) is cloned in pBI121 vector under the promoter CaMV35S, neomycin transferase II (nptII) as selectable marker, and Nos terminator.
Chemicals and reagents
Sodium bicarbonate (Himedia, catalog number: MB045), store at room temperature (RT)
Sulfuric acid (Himedia, catalog number: RM6224), store at RT
Mercuric chloride (HgCl2) (Sigma-Aldrich, catalog number: M1136), store at RT
Ethanol (Merck, catalog number: 1.00983), store at RT
Acetosyringone (Himedia, catalog number: PCT1301), store at 4 °C
Glucose (Sigma-Aldrich, catalog number: G7021), store at RT
Sucrose (Sigma-Aldrich, catalog number: S0389), store at RT
Phytagel (Sigma-Aldrich, catalog number: P8169), store at RT
Agar powder (Himedia, catalog number: MB053), store at RT
Myo-inositol (Sigma-Aldrich, catalog number: I7508), store at RT
Magnesium chloride (Sigma-Aldrich, catalog number: M4880), store at RT
Potassium chloride (Sigma-Aldrich, catalog number: P5405), store at RT
Calcium chloride (Sigma-Aldrich, catalog number: C5670), store at RT
MES buffer (Sigma-Aldrich, catalog number: M3671), store at RT
Murashige & Skoog basal salt (Sigma-Aldrich, catalog number: M5524), store at 4 °C
Potassium nitrate (Sigma-Aldrich, catalog number: P8291), store at RT
Magnesium sulfate (Sigma-Aldrich, catalog number: M2773), store at RT
Potassium phosphate monobasic (Sigma-Aldrich, catalog number: P5655), store at RT
Calcium nitrate tetrahydrate (Sigma-Aldrich, catalog number: C2786), store at RT
Ammonium phosphate monobasic (Sigma-Aldrich, catalog number: 216003), store at RT
Calcium chloride dehydrate (Sigma-Aldrich, catalog number: C7102), store at RT
Sodium molybdate dehydrate (Sigma-Aldrich, catalog number: M1651), store at RT
Boric acid (Sigma-Aldrich, catalog number: B7901), store at RT
Potassium iodide (Sigma-Aldrich, catalog number: 221945), store at RT
Manganese sulfate tetrahydrate (Sigma-Aldrich, catalog number: 1.02786), store at RT
Zinc sulfate (Sigma-Aldrich, catalog number: Z0251), store at RT
Copper sulfate pentahydrate (Sigma-Aldrich, catalog number: C8027), store at RT
Cobalt chloride hexahydrate (Sigma-Aldrich, catalog number: C8661), store at RT
Sodium EDTA (Sigma-Aldrich, catalog number: 03650), store at RT
Ferrous sulfate heptahydrate (Sigma-Aldrich, catalog number: F8633), store at RT
Nicotinic acid (Sigma-Aldrich, catalog number: N0761), store at RT
Pyridoxine HCl (Sigma-Aldrich, catalog number: P6280), store at RT
Thiamine HCl (Sigma-Aldrich, catalog number: T1270), store at RT
Kinetin (Sigma-Aldrich, catalog number: K3375), store at 4 °C
2,4-Dichlorophenoxyacetic acid (2,4-D) (Sigma-Aldrich, catalog number: D76724), store at 4 °C
6-Benzylaminopurine (BAP) (Sigma-Aldrich, catalog number: B3408), store at 4 °C
Dimethyl sulfoxide (DMSO) (Sigma-Aldrich, catalog number: D8418), store at RT
Methanol (Sigma-Aldrich, catalog number: 34860), store at RT
Sodium hydroxide (NaOH) (Sigma-Aldrich, catalog number: 221465), store at RT
Yeast extract (Sigma-Aldrich, catalog number: Y1625), store at RT
Beef extract (Himedia, catalog number: RM002V), store at RT
Peptone (Sigma-Aldrich, catalog number: P5905), store at RT
Murashige & Skoog basal salt mixture (4.3 g/L) (Sigma-Aldrich; catalog number: M2909)
Antibiotics (see Recipes)
Kanamycin 50 mg/L (Himedia, catalog number: MD026), store at 4 °C
Streptomycin 250 mg/L (Himedia, catalog number: MD048), store at 4 °C
Rifampicin 50 mg/L (Himedia, catalog number: MD045), store at 4 °C
Augmentin 250 mg/L (Himedia, catalog number: PCT1115), store at 4 °C
Cefotaxime 250 mg/L (Himedia, catalog number: MD015), store at 4 °C
Phytohormones (see Recipes)
2,4-D (stock: 10 mg/mL; working: 0.5 mg/mL)
Kinetin (stock: 10 mg/mL; working: 0.2 mg/mL)
BAP (stock: 10 mg/mL; working: 0.2 mg/mL)
Acetosyringone (see Recipes)
YEB media (see Recipes)
Induction media (see Recipes)
Hoagland media (see Recipes)
Murashige & Skoog stock (see Recipes)
Culture media (see Recipes)
Equipment
Incubator/shaker (Kuhner, model: ISF-1-W)
Laminar flow hood
Autoclave (TOMY, model: ES-315)
Ultracentrifuge (Eppendorf, model: 5430R)
pH meter (Eutech, pH tutor)
Magnetic stirrer (Abdos, Swirl top)
Thermal cycler (Bio-Rad, C1000 thermal cycler)
-80 °C ultra-low temperature freezer (Thermo Scientific, Forma 89000 Series)
-20 °C low temperature freezer (Celfrost)
Weighing balance (Sartorius, SQP-F)
Electroporation machine (MicroPluserTM Bio-Rad)
Greenhouse
Culture room
Soil mix for plants
Procedure
Transformation of Agrobacterium and seed germination for transformation
Fresh, uniform culture of transformed Agrobacterium is required for efficient plant transformation.
LBA4404 competent cells’ transformation with pBI121 (14.7 kb) construct (Figure 2):
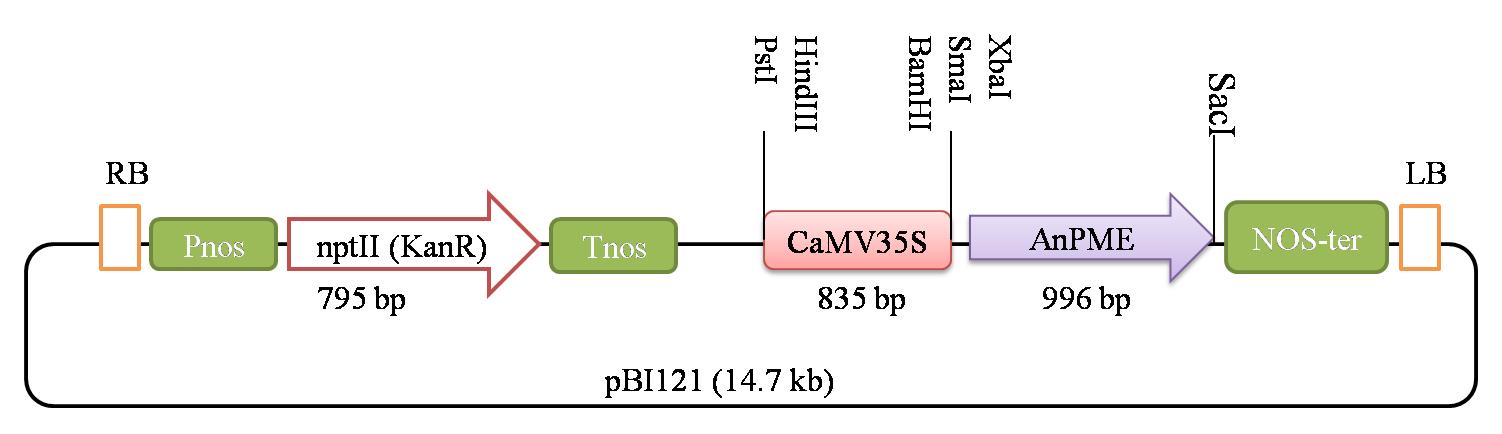
Figure 2. Construct map of the vector used for Agrobacterium-mediated genetic transformationPlace the competent cells (LBA4404) on ice and let them thaw (do not rub the Eppendorf to force thawing). Once the cells thaw, add 1 μL of the desired plasmid to the competent cells. (Plasmid concentration: 10–100 ng/μL.)
Mix the plasmid and cells by gentle pipetting and let it sit for 5 min.
Transfer the cell mixture to an electroporation cuvette and mix by gentle pipetting.
Place the cuvette into the cuvette chamber and start the electroporation machine. Press the pulse button until a beep sound is heard, indicating that the pulse has been given.
Remove the cuvette, transfer the entire cell mixture into a 1.5 mL Eppendorf, and add 950 μL of YEB media (see Recipes).
Gently mix the cell mixture by pipetting and then place it in the incubator shaking at 200 rpm for 2 h in the dark at 28 °C. Dark conditions help the bacteria to repair and prepare itself for growth and multiplication.
After 2 h, remove the Eppendorf and centrifuge at 3,500× g for 2 min at RT.
Discard the supernatant (used YEB media) and add 300 μL of YEB media again. Mix the pelleted cell mixture by pipetting.
Once mixed properly, spread 50–100 μL of cells on YEB agar plates containing 50 mg/L kanamycin, 250 mg/L streptomycin, and 50 mg/L rifampicin. Spreading should result in a moistureless plate (this leads to appropriate single colony formation).
Wrap the plates with foil and place them in an incubator at 28 °C for 48 h in the dark.
After 48 h, single colonies start to appear. Pick 10–12 single colonies and check by PCR if the cells are transformed.
Create a master plate by streaking the positive colonies on YEB agar plates containing 50 mg/L kanamycin, 250 mg/L streptomycin, and 50 mg/L rifampicin. These Agrobacterium colonies consist of our gene of interest.
Incubate the master plate for 48 h in an incubator at 28 °C.
Store the plate in the dark at 4 °C.
Cotton seed sterilization and germination (Figure 3):
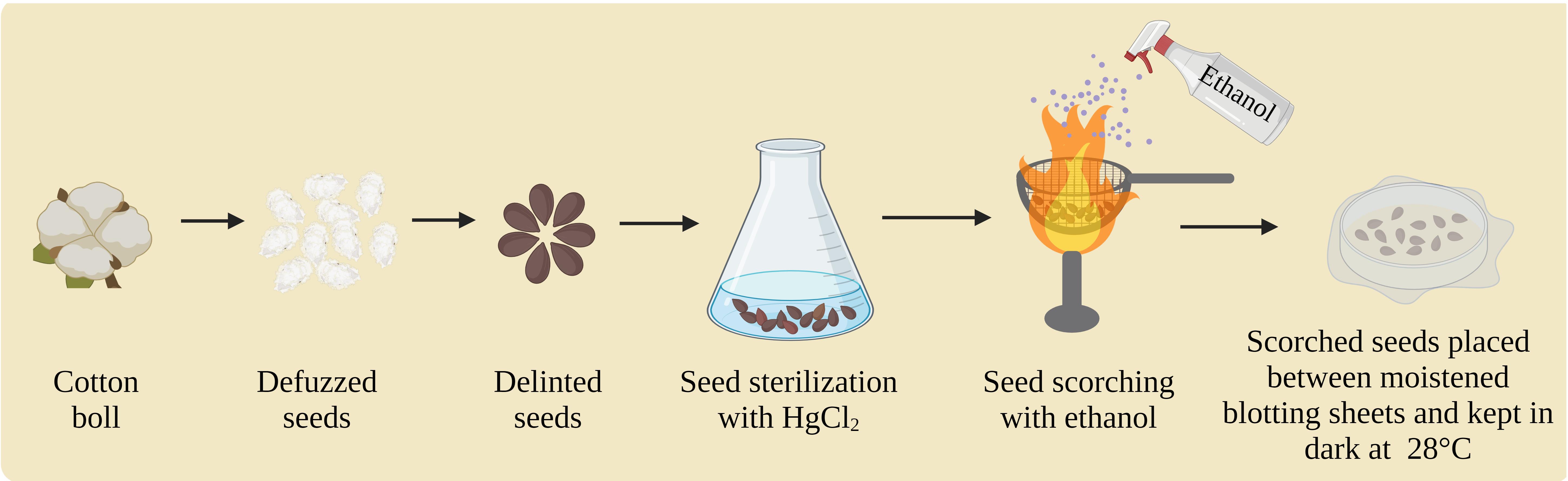
Figure 3. Cotton seed sterilization and germination (created with BioRender.com)Defuzz the cotton bolls. After defuzzing, remove the remaining lint by delinting the seeds.
To delint 100 seeds, prepare a liter of saturated sodium bicarbonate solution by mixing sodium bicarbonate in 1 L of double-distilled water until it stops dissolving.
Add 25 seeds to a 100 mL wide-mouth bottle and pour 7–8 drops of concentrated sulfuric acid on it. Stir the seeds continuously until the whole lint is removed.
Once the lint is removed, add saturated sodium bicarbonate solution to the bottle and simultaneously keep stirring the seeds. Stir the seeds until the effervescence ceases.
Discard the solution and rinse the seeds with tap water by vigorous shaking. Repeat this 4–5 times to remove traces of sodium bicarbonate.
Once washed properly, place the seeds on a blotting sheet and let them dry.
For sterilization, prepare a 0.1% (w/v) solution of HgCl2 by mixing 100 mg of HgCl2 with 100 mL of autoclaved double-distilled water in a 250 mL conical flask.
In a laminar flow hood, add 25–30 dried seeds to 0.1% HgCl2 solution and stir vigorously for 5 min. Try not to exceed 5 min. Excess sterilization will degrade the seed quality. Discard the solution and rinse the seeds 5–6 times for 30 s each by adding 100 mL of double-distilled water to the flask after every rinse.
After sterilizing the seeds, place half in a stainless-steel strainer and scorch them with ethanol. Do not burn the seeds. Scorching loosens the seed coat and aids in better germination (Video 1).
Video 1. Seed scorchingLet the seeds cool down for 5 min.
Cut two pieces of filter paper in the shape of a Petri plate (90 × 15 mm). Pour autoclaved double-distilled water, just enough to dampen the filter paper. Next, place the seeds on it and cover them with cut pieces of filter paper. Once again, pour enough autoclaved double-distilled water to dampen it.
Seal the Petri plate with parafilm tape and cover it with foil. Place the plate in an incubator at 28 °C for 48–72 h.
Radicals start appearing within two days. Once proper radicals are seen, place them on paper boats (made of computer paper) in test tubes containing Hoagland media (see Recipes, Table 1), with the radical in a downward position. Seeds can also be grown on ½ MS media, but Hoagland media is preferred as its carbohydrate absence leads to fewer chances of contamination.
Place the test tubes in the test tube stand and keep the stands in a culture room set at 28 °C.
Plantlets appear in 10 days, ready to be used for transformation (Figure 4).

Figure 4. Seed germination. a) Test tube with a paper boat immersed in Hoagland media, on which a cotton plantlet has been grown. b) Fully grown 10-day-old plantlet with a slender and proper hypocotyl. c) 10-day-old plantlets grown in ½ Murashige & Skoog media with phytagel, ready for transformation (shown in red box).
Plant transformation and transgenic development
Agrobacterium culture preparation and induction for plant transformation (Figure 5):
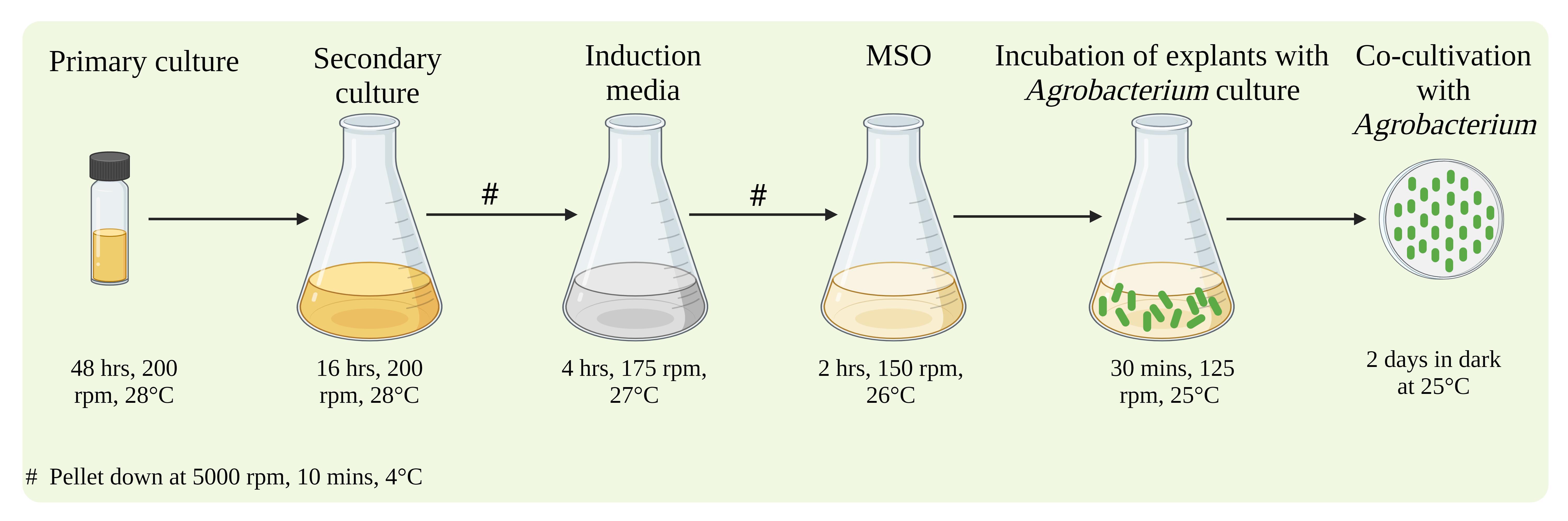
Figure 5. Agrobacterium culture preparation and induction for plant transformation (created with BioRender.com)Using a toothpick, pick a single colony from the master plate and add to it 5 mL of YEB media (with 50 mg/L kanamycin, 250 mg/L streptomycin, and 50 mg/L rifampicin) in a 30 mL culture vial. Incubate the culture for 2–3 days in an incubator with shaking at 200 rpm at 28 °C. This primary will be used for culturing a secondary culture.
After the culture grows in 5 mL of YEB media, take 100 μL of culture and inoculate it in 50 mL of YEB media containing 50 mg/L kanamycin, 250 mg/L streptomycin, and 50 mg/L rifampicin in a 250 mL conical flask. Incubate it for 16 h with shaking at 200 rpm at 28 °C.
Check the OD of the secondary culture after 16 h. A culture OD600 = 0.8–1.6 is used for transformation.
Once the desired OD is attained, transfer the cultures to 50 mL falcon tubes. Use two autoclaved 50 mL falcons for 50 mL of culture, equally dividing 25 mL into each falcon. Pellet down the cells at 3,500× g for 10 min at 4 °C.
Discard the supernatant and dissolve the pellets, each in 50 mL of induction media, and transfer the total 100 mL of media to a 250 mL conical flask. Incubate for 4 h with shaking at 175 rpm at 27 °C.
After 4 h, centrifuge the culture at 3,500× g for 10 min at 4 °C. Dissolve the pellet in 100 mL of Murashige & Skoog-0 (MSO; see Recipes, Table 3) media and incubate for 2 h with shaking at 150 rpm at 26 °C. Later on, this culture will be used for transformation.
Plant transformation:
In a laminar flow hood, take the hypocotyls from the 10-day-old plantlet and cut them into 1 cm long pieces. After cutting, place them on a plain MS media plate, so as not to dry them out (Video 2).
Video 2. Explant preparationMeanwhile, the Agrobacterium culture should be ready in MSO for further transformation processes. Add the explants to 100 mL of MSO and incubate the mixture of explants and culture at 25 °C with shaking at 125 rpm for 30 min.
Remove the explants from the culture after 30 min and blot them dry with an autoclaved filter paper sheet.
Arrange the explants on MSP-1 culture media (without any antibiotics; see Recipes, Table 3) for co-cultivation. Wrap the plates with foil and keep in the dark for 48 h.
After 48 h, wash the explants with 100 mL of autoclaved double-distilled water in a conical flask containing 250 mg/L cefotaxime. Shake the flask for 5 min and then rinse 4–5 times again with autoclaved water.
Once the explants are properly washed, dry them on an autoclaved filter paper.
Callus induction and somatic embryogenesis (Figure 6):

Figure 6. Regeneration from transformed explants and transgenic development. a) Callus induction. b) Callus initiation. c) Callus proliferation. d) Embryogenic callus. e) Germinated embryo in ½ Murashige & Skoog with phytagel media. f) Developed plantlet. g) Hardening of plantlets. h and i) Fully grown transgenic plants.After drying the explants, place 8–9 explants on each selection media (MSP-1; see Recipes, Table 3) plate. Place the culture plates in a culture room set at 28 °C with 16 h light and 8 h dark conditions.
Subculture the explants after 21 days on selection media. At this stage, callus starts appearing from the ends of the explants.
After two cycles of MSP-1, place the explants on MSP-2 (see Recipes, Table 3) media for a third and fourth cycle. A total of four cycles (each consisting of 21 days) of antibiotics ensure proper selection of positive callus.
At this point, the calli starts proliferating, and lots of friable, yellow-colored beady calli can be seen. Remove the callus from the explants with caution and place it on MSA-1 (see Recipes, Table 3) for further proliferation. Subculture the callus every 21 days for an extra 2–3 times. Lots of yellowish-colored, friable calli emerge when proper subculturing is done. Embryogenic callus will have a tinge of pinkish color, which is due to presence of the anthocyanin pigment. Presence of pink color shows onset of embryogenesis (Figure 7).
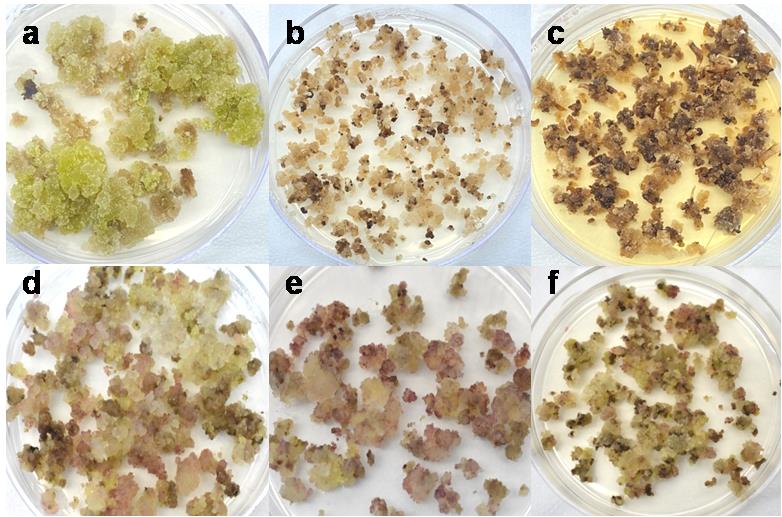
Figure 7. Different types of regenerated callus. a–c) Non-viable calli can be green, light brown, or dark brown in color. These types of calli are either too hard (a) or soggy and wet (b and c). d–f) Viable calli, which are friable, beady, and have a slight pinkish color.Transfer the friable callus to MSA-2 (see Recipes, Table 3) media for embryogenesis. Normal friable callus shows embryogenesis on plain MS media too, but the absence of NH4NO3 results in more anthocyanin production, which is an indication of stress for plants. As such, embryogenesis starts early in callus. Also, placing the proliferating callus at lower temperature (2–3 °C) accelerates the embryogenesis process.
Once the globular embryos can be seen converting to bipolar (Figure 8), subculture them on ½ MSP (see Recipes, Table 3) plates. When placed on ½ MSP, the root develops appropriately, giving the required anchorage for growth (Video 4).
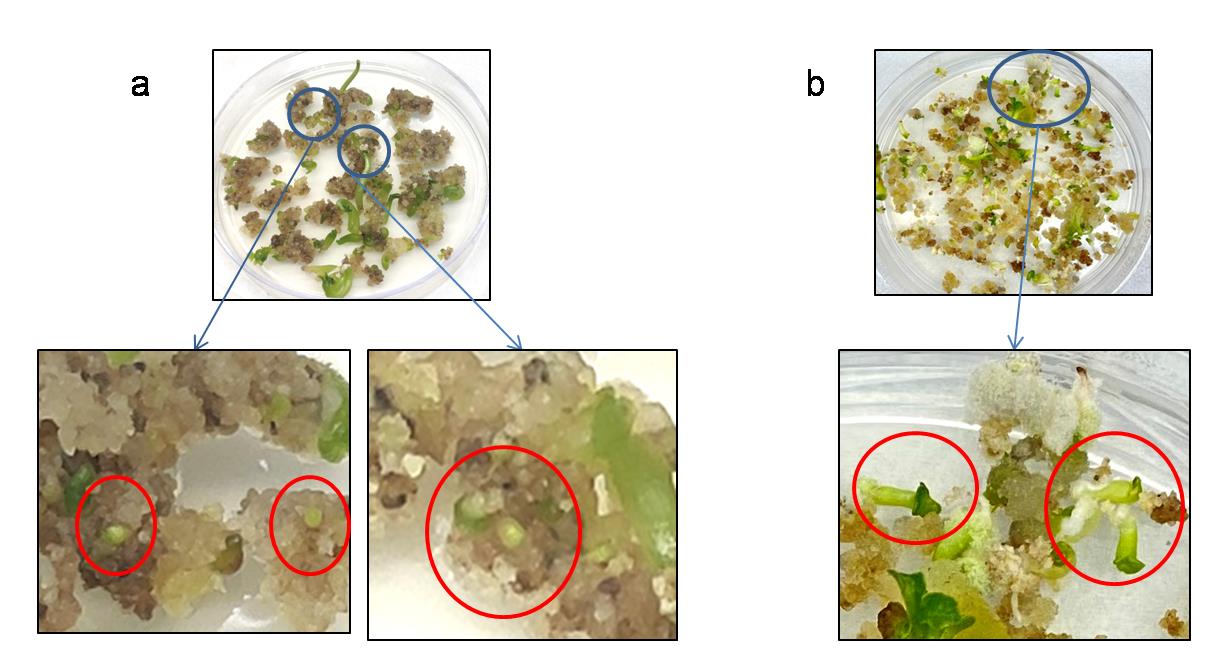
Figure 8. Distinguishing stages of embryos. a) Globular embryo development after a few subcultures. b) Development of bipolar embryos. Once developed, bipolar embryos leave the connection with callus and have their own root and shoot apex with vascular connections.Remove the bipolar embryos from the plates after 10 days and place them in planton boxes containing approximately 100 mL of ½ MSP media. This ensures the required space for plantlet development.
Let the plantlets grow a decent and stable root system. During this time interval, the shoot system starts touching the planton box cap, so replace the cap with another autoclaved planton box, attaching both the boxes with a coupler frame.
Transfer the developed transgenic plant into a 5” plastic pot containing autoclaved soil mixture, and water it with Hoagland media (see Recipes). Cover the pot with a polythene bag. Sprinkle a few drops of water inside the bag to maintain moisture.
Place the pots in a plastic tray and pour Hoagland media in the tray to maintain the water level in the pots.
Transfer these pots to the culture room. After 10 days, shift them to a greenhouse for hardening. One week later, make a cut at the top of polythene bag. After another week, remove the polythene bag from the pots. This ensures appropriate acclimatization of transgenic plants.
After 10–15 days, when the plants are 15–20 cm long, transfer them into 14” earthen pots containing cotton field soil. Place them in a greenhouse at 28 °C until the harvest.
Extract DNA from the plants and conduct PCR to confirm the insertion of the gene of interest in the cotton plants (Figure 9).
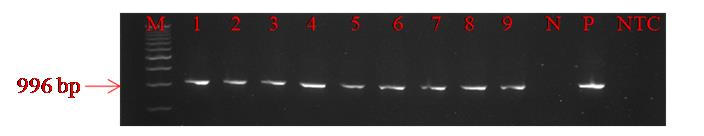
Figure 9. PCR check for the presence of the AnPME gene in putative transgenic lines. 1–9: transgenic lines; N: negative control; P: positive control; NTC: non-template control.
Notes
A few drops of sulfuric acid are sufficient for delinting. Adding excess sulfuric acid will damage the seeds. Dry the seeds properly. Keep the seeds in a 30 °C incubator for several hours before use.
Adding antibiotics to hot media will break down the antibiotics. Therefore, check the temperature of the media before adding antibiotics; it should be bearable when touched with a bare hand.
Bacterial cell suspension culture density must be between 0.8 and 1.6. OD at 600 nm. A lower cell density results in no transformation at all; higher cell density leads to increased Agrobacterium contamination after/during co-cultivation. This causes rotting of the explants. OD600 = 1.2 is best suited for transformation.
Using fresh secondary culture is recommended for transformation. The cell culture density must be attained overnight. Cultures growing for 24–48 h to get the desired OD, or cultures exceeding the recommended point that are further diluted to use for transformation purposes do not ensure efficient transformation.
Augmentin (250 mg/L) ceases the Agrobacterium proliferation after transformation. Therefore, adding it to the selection media ensures efficient transformation.
Callus subculturing is the crucial step in cotton somatic embryogenesis. Yellowish, beady, and friable calli are best suited for regeneration (Video 3). Friable calli can also appear alongside non-viable callus. Therefore, the viable calli should be separated from the non-viable ones and subcultured on a different MSA-2 plate (see Video 3). Some calli appear to be friable, but are hard from the inside when handled with forceps (Figure 7); this type of callus should be discarded. Sometimes, calli become soggy or can get contaminated; these should be removed before subculturing onto the next media.
Video 3. Viable callus subculturingEmbryogenic callus should be subcultured with care and must be separated from the normal callus. Once the embryogenic callus is segregated, embryogenesis is accelerated.
Different stages of embryos should be taken care of. Globular embryos are very small but visible via the naked eye when they appear (Figure 8); take care not to injure them during subculturing. When the embryos convert to the bipolar stage, they leave the connection with the callus and develop their own vascular system (Figure 8). At this stage, embryos can also be removed manually and placed in ½ MSP for further growth (Video 4).
Video 4. Embryo subculturingIt is recommended to transfer plantlets that have developed suitable bipolar growth to the planton boxes. Embryos transferred to ½ MSP growth medium plates may proliferate rapidly but may not have yet developed roots. We recommend using a 100 × 20 mm Petri plate to allow room for growing plantlets. Appropriate space and additional media create the right environment for plant development.
Plantlets are grown in culture media where phytagel is used as a hardening base. Phytagel ensures smooth development of the roots. It also plays an important role while transferring the plantlets to the pots for hardening, as roots can be extracted easily from the phytagel media as it is comparatively less hard than agar.
There can be abnormal embryo formation too. Gibberellic acid (GA3; 1 mg/L) can be used in the ½ MSP culture media to salvage those embryos. Adding GA3 to the media reduces the abnormality.
Precise and cautious removal of media from the roots is extremely necessary, as leftover media decays the root.
During the hardening process, sudden exposure to environmental changes should be avoided. Gradual shifting from culture room to glass house is recommended.
Recipes
Antibiotics
Kanamycin (stock: 100 mg/mL; working: 50 mg/L)
Dissolve 1 g of kanamycin powder in 9.8 mL of double-distilled water. Filter-sterilize with a 0.22 μm syringe filter and store at 4 °C in 1 mL aliquots. Use 500 μL/L in media.
Streptomycin (stock: 250 mg/mL; working: 250 mg/L)
Dissolve 1 g of streptomycin powder in 3.8 mL of double-distilled water. Sterilize with a 0.22 μm syringe filter and store in 1 mL aliquots at 4 °C. Use 1 mL/L in media.
Rifampicin (working: 25 mg/L; always prepared fresh)
Dissolve 25 mg of rifampicin powder in 1 mL of methanol and sterilize with a 0.22 μm syringe filter. Use 1 mL/L in media. Do not store.
Augmentin (working: 250 mg/L; prepared fresh)
Dissolve 1 g of augmentin powder in 3.8 mL of double-distilled water. Sterilize with a 0.22 μm syringe filter and store in 1 mL aliquots at 4 °C. Use 1 mL/L in media. Avoid storing this antibiotic.
Cefotaxime (stock: 250 mg/mL; working: 250 mg/L)
Dissolve 1 g of cefotaxime powder in 3.8 mL of double-distilled water. Sterilize with a 0.22 μm syringe filter and store in 1 mL aliquots at 4 °C. Use 1 mL/L in media.
Phytohormones
2,4-D (stock: 10 mg/mL; working: 0.5 mg/mL): Dissolve 100 mg of 2,4-Dichlorophenoxyacetic acid powder in 1 mL of ethanol and adjust to 10 mL with double-distilled water. Store at 4 °C.
Kinetin (stock: 10 mg/mL; working: 0.2 mg/mL): Dissolve 100 mg of kinetin powder in 1 mL of 1 N NaOH and adjust to 10 mL with double-distilled water. Store at 4 °C.
BAP (stock: 10 mg/mL; working: 0.2 mg/mL): Dissolve 100 mg of BAP powder in 1 mL of 1 N NaOH and adjust to 10 mL with double-distilled water. Store at 4 °C.
Acetosyringone
Dissolve 19.3 mg of acetosyringone powder in 1 mL of DMSO. Filter-sterilize it with a 0.22 μm syringe filter. Store at 4 °C and in the dark. If the solution is prepared correctly, it freezes at 4 °C. Use 100 μL in 100 mL media.
YEB media (1 L), pH = 7.2
Yeast extract 1 g
Beef extract 5 g
Peptone 5 g
Magnesium sulphate heptahydrate 500 mg
Sucrose 5 g
Agar powder 1.5%
Autoclave at 121 °C for 20 min and 15 psi
Induction media (1 L), pH = 6.0
Ammonium chloride 1 g
Magnesium sulphate heptahydrate 0.3 g
Potassium chloride 0.15 g
Calcium chloride 0.01 g
Ferrous sulphate heptahydrate 0.0025 g
Potassium phosphate monobasic 0.272 g
MES buffer 0.390 g
Acetosyringone 100 μm (added after autoclaving)
Glucose 5 g
Autoclave at 121 °C for 20 min and 15 psi
Hoagland media (1 L)
Major salts 50 mL
Minor salts 1 mL
Table 1. Media composition for Hoagland’s media
S. No. Components g/L Major salts (1 L), stock 50× 1 Potassium nitrate 121.32 2 Calcium nitrate tetrahydrate 18.9 3 Ammonium phosphate monobasic 4.6 4 Magnesium sulphate heptahydrate 4.9 Minor salts (500 mL) 1,000× 5 Potassium chloride 1.86 6 Boric acid 0.773 7 Manganese sulphate hydrate 0.169 8 Zinc sulphate heptahydrate 0.2875 9 Copper (II) sulphate 0.0625 10 Sodium molybdate 0.505 Murashige & Skoog stock preparation
All the components are mixed accordingly and stored in reagent bottles. Stocks can be stored up to one month at 4 °C. MS-III requires an amber bottle for storing.
If ammonium nitrate is not available, Murashige & Skoog basal salt mixture (4.3 g/L) can be used instead of preparing stocks. While using MS basal salt mixture, GAM vitamins need to be added additionally, equal to the quantity given in the table below. However, it is recommended to use stocks for media preparation.
Table 2. Murashige & Skoog’s media composition
S. No. Components g/L g/L (final standard) MS-I (major salts), stock 50× 1 Ammonium nitrate 82.5 1.650 2 Potassium nitrate 95.0 1.900 3 Magnesium sulphate heptahydrate 18.5 0.370 4 Potassium phosphate monobasic 8.5 0.170 MS-II (CaCl2), stock 100× 5 Calcium chloride dihydrate 44.0 0.440 MS-III (minor salts), stock 1,000× 6 Sodium molybdate 0.250 0.250 7 Boric acid 6.200 6.200 8 Potassium iodide 0.830 0.830 9 Manganese sulphate tetrahydrate 22.300 22.30 10 Zinc sulphate heptahydrate 8.6 8.6 11 Copper sulphate trihydrate 0.025 0.025 12 Cobalt chloride hexahydrate 0.025 0.025 MS-IV (Fe-EDTA), stock 200× 13 Sodium EDTA 7.460 37.3 14 Ferrous sulphate heptahydrate 5.560 27.8 MS-V (GAM Vitamin), stock 1,000× 15 Nicotinic acid 1.00 1 16 Pyridoxine HCl 1.00 1 17 Thiamine HCl 1.00 1 Culture media preparation
Prepare culture media components either in stock form or use fresh components. Even when stocks (Table 2) are used for media preparation, mix all the components accordingly and autoclave at 121 °C for 20 min and 15 psi. Once cooled, add antibiotics, give a good stir, and pour in 90 × 20 mm Petri dishes in a laminar flow hood.
Table 3. List of different media used at different stages of transformation and regeneration of cotton
Culture media (per liter) S. No. Component MSO ½ MSP MSP-1 MSP-2 MSA-1 MSA-2 1 MS major 20 mL 10 mL 20 mL 20 mL 20 mL 20 mL (without NH4NO3 and double KNO3) 2 MS minor 1 mL 0.5 mL 1 mL 1 mL 1 mL 1 mL 3 GAM vitamin 1 mL 0.5 mL 1 mL 1 mL 1 mL 1 mL 4 Calcium chloride 10 mL 5 mL 10 mL 10 mL 10 mL 10 mL 5 Fe-EDTA 5 mL 2.5 mL 5 mL 5 mL 5 mL 5 mL 6 Myo-inositol 100 mg 100 mg 100 mg 100 mg 100 mg 7 Glucose 10 g 30 g 30 g 30 g 30 g 30 g 8 Magnesium chloride 750 mg 750 mg 750 mg - - 9 BAP - 0.2 mg - - - 10 Kinetin - 0.2 mg - - - 11 2,4-D - 0.5 mg - - - 12 Phytagel 2.2 g 2.2 g 2.2 g - - 13 Agar - - - 8 g 8 g 14 Kanamycin - 50 mg 50 mg - - 15 Augmentin - 250 mg 250 mg - - 16 MES buffer 1.950 g - - - - - 17 pH 6.5 5.8 5.8 5.8 5.8 5.8 MSO: Murashige & Skoog-0 media
MSP: Murashige & Skoog with phytagel
MSA: Murashige & Skoog with agar
Acknowledgments
We are grateful to Scientific and Engineering Board (SERB), India for providing us research grant (CRG/2021/005998) to support our research work. We are also thankful to Council of Scientific and Industrial Research (CSIR), India for providing the research facilities. This protocol has been derived from our previous research paper by Shukla et al. (2016). Institutional Manuscript ID No. CSIR-NBRI_MS/2023/02/02.
Competing interests
The authors declare that there are no existing conflicts of interest as to their knowledge.
References
- Aydin, Y., Ipekci, Z., Talas-Oğraş, T., Zehir, A., Bajrovic, K. and Gozukirmizi, N. (2004). High Frequency Somatic Embryogenesis in Cotton. Biol Plant 48(4): 491-495.
- Bajwa, K. S., Shahid, A. A., Rao, A. Q., Bashir, A., Aftab, A. and Husnain, T. (2015). Stable transformation and expression of GhEXPA8 fiber expansin gene to improve fiber length and micronaire value in cotton.Front Plant Sci 6: 838.
- Finer, J. J. (1988). Plant regeneration from somatic embryogenic suspension cultures of cotton (Gossypium hirsutum L.). Plant Cell Rep 7(6): 399-402.
- Firoozabady, E., DeBoer, D. L., Merlo, D. J., Halk, E. L., Amerson, L. N., Rashka, K. E. and Murray, E. E. (1987). Transformation of cotton (Gossypium hirsutum L.) by Agrobacterium tumefaciens and regeneration of transgenic plants. Plant Mol Biol 10(2): 105-116.
- Ikram-ul-Haq and Zafar, Y. (2004). Effect of nitrates on embryo induction efficiency in cotton (Gossypium hirsutum L.). Afr J Biotechnol 3(6): 319-323.
- Hemphill, J. K., Maier, C. G. A. and Chapman, K. D. (1998). Rapid in-vitro plant regeneration of cotton ( Gossypium hirsutum L.).Plant Cell Rep 17(4): 273-278.
- Jadhav, M. P. and Katageri, I. S. (2017). Agrobacterium tumefaciens Mediate Genetic Transformation in Coker-312 (Gossypium hirsutum L.) Using Hypocotyls Explants. Int J Curr Microbiol App Sci 6(12), 2771-2779.
- Jin, S., Zhang, X., Liang, S., Nie, Y., Guo, X. and Huang, C. (2005). Factors affecting transformation efficiency of embryogenic callus of Upland cotton (Gossypium hirsutum) with Agrobacterium tumefaciens. Plant Cell Tissue Organ Cult 81(2): 229-237.
- Jones, H. D., Doherty, A. and Wu, H. (2005). Review of methodologies and a protocol for the Agrobacterium-mediated transformation of wheat. Plant Methods1(1): 5.
- Kumar, M. and Tuli, R. (2004). Plant regeneration in cotton: A short-term inositol starvation promotes developmental synchrony in somatic embryogenesis. In Vitro Cell Dev Biol Plant 40(3): 294-298.
- Manickavasagam, M., Ganapathi, A., Anbazhagan, V. R., Sudhakar, B., Selvaraj, N., Vasudevan, A. and Kasthurirengan, S. (2004). Agrobacterium-mediated genetic transformation and development of herbicide-resistant sugarcane (Saccharum species hybrids) using axillary buds. Plant Cell Rep 23(3): 134-143.
- McCabe, D. E. and Martinell, B. J. (1993). Transformation of Elite Cotton Cultivars via Particle Bombardment of Meristems. Bio/Technology 11: 596-598.
- Pathi, K. M. and Tuteja, N. (2013). High-frequency regeneration via multiple shoot induction of an elite recalcitrant cotton (Gossypium hirsutum L. cv Narashima) by using embryo apex.Plant Signal Behav 8(1): e22763.
- Qandeel-E-Arsh, Azhar, M. T., Atif, R. M., Israr, M., Khan, A. I., Khalid, S. and Rana, I. A. (2021). A discussion on cotton transformation during the last decade (2010–2021); an update on present trends and future prospects.J Cotton Res 4(1): 29.
- Satyavathi, V. V., Prasad, V., Gita Lakshmi, B. and Lakshmi Sita, G. (2002). High efficiency transformation protocol for three Indian cotton varieties via Agrobacterium tumefaciens. Plant Sci 162(2): 215-223.
- Shoemaker, R. C., Couche, L. J. and Galbraith, D. W. (1986). Characterization of somatic embryogenesis and plant regeneration in cotton (Gossypium hirsutum L.). Plant Cell Rep 5(3): 178-181.
- Shukla, A. K., Upadhyay, S. K., Mishra, M., Saurabh, S., Singh, R., Singh, H., Thakur, N., Rai, P., Pandey, P., Hans, A. L., et al. (2016). Expression of an insecticidal fern protein in cotton protects against whitefly. Nat Biotechnol 34(10): 1046-1051.
- Somleva, M. N., Tomaszewski, Z. and Conger, B. V. (2002). Agrobacterium -Mediated Genetic Transformation of Switchgrass.Crop Sci 42(6): 2080-2087.
- Sun, Y., Zhang, X., Huang, C., Guo, X. and Nie, Y. (2006). Somatic embryogenesis and plant regeneration from different wild diploid cotton (Gossypium) species. Plant Cell Rep 25(4): 289-296.
- Wang, M., Zhang, B. and Wang, Q. (2013). Cotton transformation via pollen tube pathway. Methods Mol Biol 958: 71-77.
- Ziemienowicz, A. (2014). Agrobacterium-mediated plant transformation: Factors, applications and recent advances.Biocatal Agric Biotechnol 3(4): 95-102.
Article Information
Publication history
Published: May 20, 2023
Copyright
© 2023 The Author(s); This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/).
How to cite
Srivastava, A., Shukla, A. K., Srivastava, S., Dubey, R. S., Singh, P. K. and Verma, P. C. (2023). Agrobacterium-mediated Genetic Transformation of Cotton and Regeneration via Somatic Embryogenesis. Bio-protocol 13(10): e4677. DOI: 10.21769/BioProtoc.4677.
Category
Plant Science > Plant transformation > Agrobacterium
Molecular Biology > DNA > Transformation
Biological Sciences > Biological techniques > Microbiology techniques
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Tips for asking effective questions
+ Description
Write a detailed description. Include all information that will help others answer your question including experimental processes, conditions, and relevant images.
Share
Bluesky
X
Copy link










