- Submit a Protocol
- Receive Our Alerts
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
A Large-format Polyacrylamide Gel with Controllable Matrix Mechanics for Mammalian Cell Culture and Conditioned Media Production
Published: Vol 13, Iss 17, Sep 5, 2023 DOI: 10.21769/BioProtoc.4807 Views: 408
Reviewed by: Masashi AsaiXin XuAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols
Production of Recombinant Hepatitis B virus (HBV) and Detection of HBV in Infected Human Liver Organoids
Tanvir Hossain [...] Tokameh Mahmoudi
Apr 20, 2022 1829 Views
Low-viscosity Matrix Suspension Culture for Human Colorectal Epithelial Organoids and Tumoroids
Tao Tan [...] Oliver M. Sieber
Apr 20, 2022 2231 Views
Three-dimensional Co-culture Model for Live Imaging of Pancreatic Islets, Immune Cells, and Neurons in Agarose Gel
Elke M. Muntjewerff [...] Gustaf Christoffersson
Oct 20, 2023 707 Views
Abstract
Tissue culture plastic has been used for routine cell culture and in vitro experiments for over 50 years. However, cells are mechanically responsive and behave differently on hard surfaces than they do on softer substrates. Polyacrylamide gels have become a popular hydrogel of choice for controlling surface stiffness and ligand density for cell adhesion. Many synthesis methods use coverslips and small gel surface areas for cell culture, which are amenable to microscopy-based experiments. However, none of the currently published methods can be scaled up to increase the surface area to accommodate conditioned media production, high volume analyte collection, or cell line expansion. To overcome this size limitation, we developed a protocol for synthesizing polyacrylamide in glass dishes using commercially available materials. This enables routine cell culture on soft surfaces and facilitates experiments that require large amounts of analyte, especially studies involving extracellular vesicles and secreted factors.
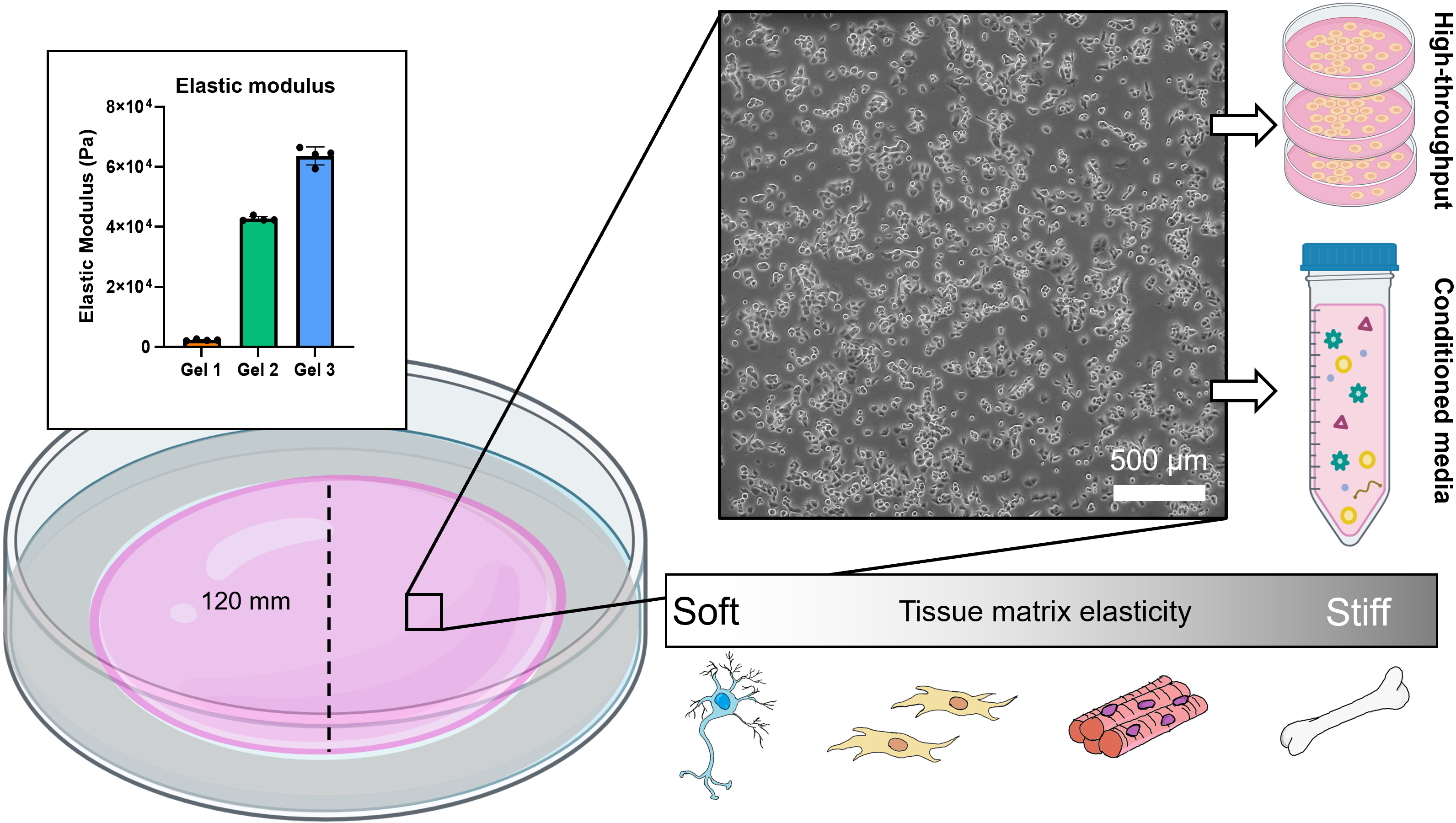
Graphical overview
Background
Culturing mammalian cells on plastic is regarded as the gold standard—it is quick, easy, and inexpensive. However, the mechanical properties of plastic surfaces are not physiological, and many studies have demonstrated phenotypic, secretory, and transcriptional differences in cells cultured on compliant surfaces. Most tissues in the body are soft and have an elastic modulus (E) that falls between 1 and 100 kPa (Engler et al., 2006;Chandler et al., 2011;Gleghorn et al., 2013). Plastic is rigid and its elastic modulus measures on the order of Gigapascals (Chen and Simmons, 2011). Some cell lines are more sensitive to mechanical stimuli than others. Well established cell lines that have been cultured on plastic for many generations (e.g., NIH 3T3 fibroblasts) are less likely to be affected by routine culture as they have already been conditioned to grow on hard surfaces. Primary cells, which are isolated directly from fresh tissue, are susceptible to undesirable phenotypic and genotypic changes when they are placed in these altered mechanical environments. When cultured on plastic, cells may transition to an activated or inflammatory state, de-differentiate, or lose their primary function. Primary cells are thus passage-limited and have a relatively short expansion window before new cells must be used.
Matrix mechanics directly modulate cell signaling and function (Nelson and Gleghorn, 2012; Millar-Haskell et al., 2019; Morgan et al., 2019; Trompeter et al., 2021). This has been observed from in vitro experiments using a variety of hydrogels of different compositions and structure. Polyacrylamide gels are particularly useful because they are elastic, the stiffness can be tightly controlled by varying the concentrations of prepolymer, and a variety of extracellular matrix proteins can be conjugated to the gel for cell attachment. Polyacrylamide gel systems have yielded important observations regarding durotaxis (Lachowski et al., 2017), mechanotransduction (Stanton et al., 2019), and differentiation (Engler et al., 2006). Over the course of a decade, protocols have been published detailing the creation of polyacrylamide coverslips or platforms for cell-based experiments (Aratyn-Schaus et al., 2010;Tse and Engler, 2010; Mih et al., 2011; Syed et al., 2015; Kumai et al., 2021). These protocols present a standardized methodology, allowing experiments across research groups to be compared more easily. Generally, the process involves functionalizing a glass surface (e.g., coverslips or multi wells) to facilitate polyacrylamide attachment during polymerization. Sulfo-SANPAH is used to conjugate an extracellular matrix protein of choice to the polyacrylamide gel for cell attachment. Cells are then seeded on top of these gel surfaces for 24–72 h to study morphological and phenotypic changes. Polyacrylamide gel coverslips are useful for high resolution fluorescent and traction force microscopy and enable experiments involving single cell tracking. Modifications to the method can give rise to patterned surfaces and custom stiffness gradients (Tse and Engler, 2010). However, these protocols are not adaptable for routine cell culture or experiments that require large surface areas due to the inherently small sizes of the platforms. Additionally, they are not amenable to conditioned media generation for studying the release of secreted factors, RNA, extracellular vesicles, and other media components (Millar‐Haskell et al., 2022a). We focused on overcoming this size limitation to enable greater versatility in polyacrylamide gel platforms.
We have developed a method of synthesizing polyacrylamide gels inside glass dishes so that they can be used like normal tissue culture dishes (Figure 1). The process is much like casting a gel for polyacrylamide gel electrophoresis, except the gel is polymerized horizontally in a glass cell culture dish. The materials used in this protocol are easy to obtain, autoclave-safe, and re-usable. The polydimethylsiloxane (PDMS) ring and acrylic cover create a seal during polymerization, and the cover is removed after gelation. This format prevents the introduction of excess oxygen during polymerization and creates a flat surface for cell culture. Additionally, this protocol uses 145 mm glass dishes, but it can be adapted for any size, including traditionally used 100 mm glass dishes or, in cases where a custom shape is needed, by changing the dimensions of the PDMS ring. These dishes can be made in bulk and stored for later use as needed. We found that we could culture our cells (PANC-1 pancreatic cancer cells) in these dishes for up to a week with no loss of viability and minimal cell damage upon detaching them (Figure 2). Possible applications of this platform include conditioned media production, supernatant analysis (e.g., extracellular vesicle release or protein secretion), or large cell lysate collection for downstream analysis (Millar‐Haskell et al., 2022b).
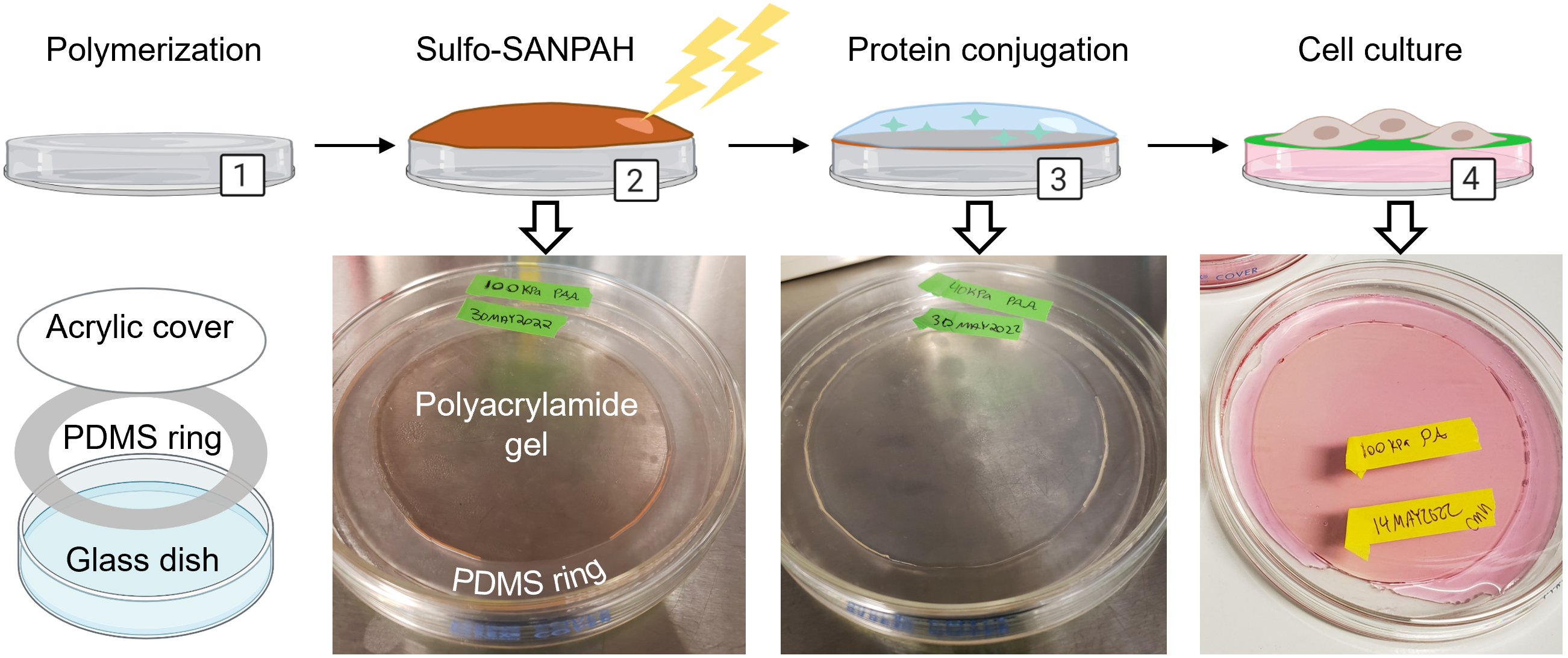
Figure 1. Overview for creating polyacrylamide dishes. (1) Polyacrylamide is synthesized in a glass dish using a PDMS ring and acrylic cover. (2) The crosslinker sulfo-SANPAH is activated on the polyacrylamide with UV light and the excess is rinsed off. (3) Extracellular matrix protein is diluted in buffer and incubated on the polyacrylamide to initiate conjugation. (4) After soaking dishes in HBS to remove unconjugated protein, dishes are pre-incubated with media and are ready for cell culture. Created with Biorender.com. Adapted from Millar-Haskell et al. (2022b).
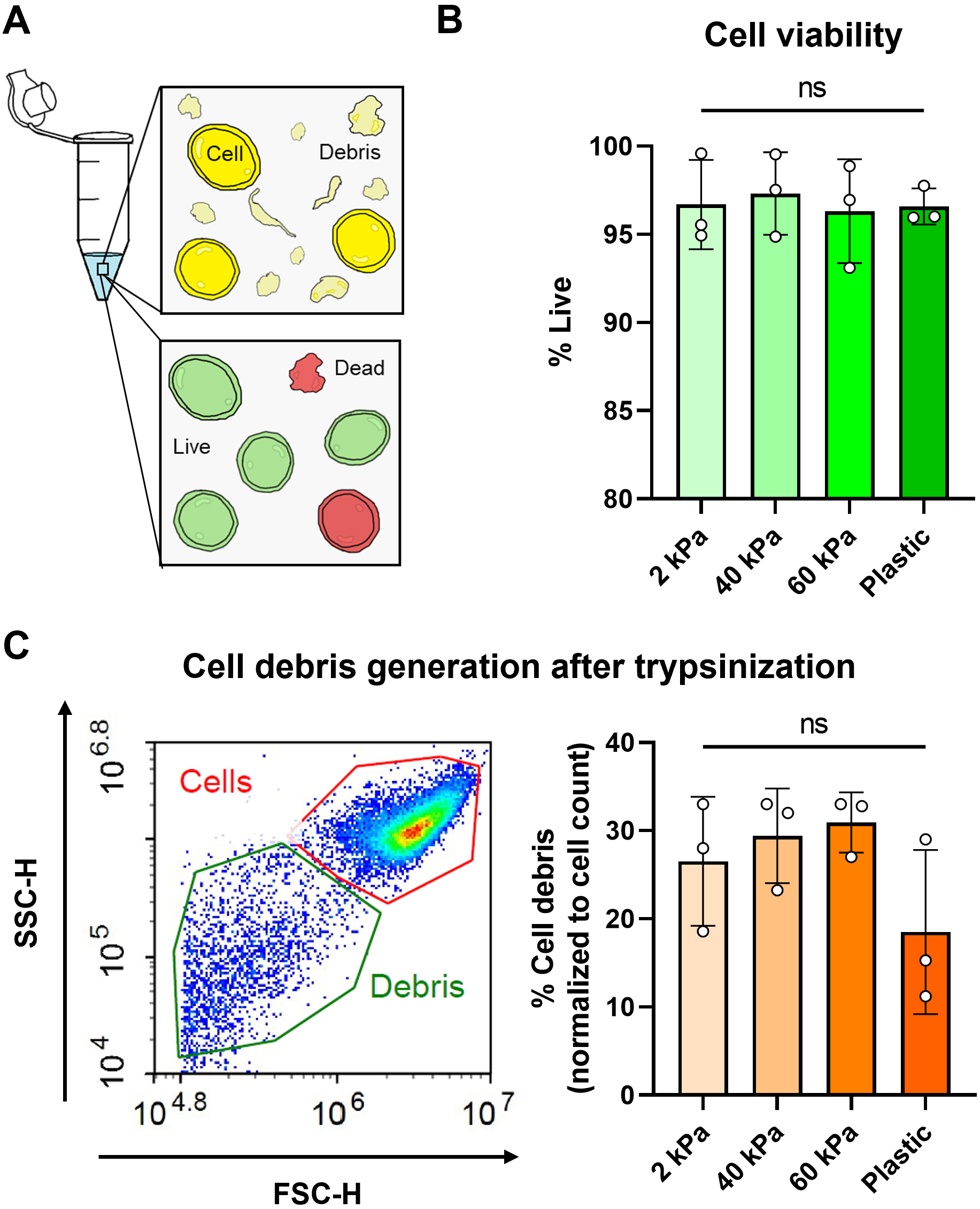
Figure 2. Cell viability assessment using calcein AM dye and flow cytometry. (A) Cartoon representation of cells in a tube after trypsinization with the presence of debris caused by cell death/compromised membranes. Cells were also stained using a live/dead viability assay. (B) Viability of PANC-1 cells cultured on polyacrylamide gel dishes of different elastic modulus and tissue culture plastic. Viability was quantified by percentage of calcein AM+ cells using flow cytometry. (C) Cell debris generation (which corresponds to cell damage and death) was normalized as a percentage of total cell count. Example plot with cell and debris gating is shown.
Materials and reagents
15 mL conical tubes (CELLTREAT, catalog number: 667016B)
Cleanroom wipes (Fisher Scientific, Contec, catalog number: 19-130-5932)
Plastic spoons and clear plastic cups (recommend Amazon or any major retailer)
Feather scalpel blades #11 (Electron Microscopy Sciences, catalog number: 72044-11)
Sylgard 184 silicone elastomer kit (Krayden, catalog number: DC4019862)
145 mm glass Petri dishes (VWR, catalog number: 25354-127)
145 mm plastic Petri dishes (VWR, catalog number: 82050-600)
0.2 μm PES bottle top filter (VWR, catalog number: 10040-436)
Autoclavable sterilization pouches (VWR, Cardinal Health, catalog number: 11213-033)
Sodium hydroxide (0.1 M NaOH)
Sodium chloride (NaCl) (Sigma-Aldrich, catalog number: S9888)
3-aminopropyltriethoxysilane (APTES) (Fisher Scientific, ACROS organics, catalog number: AC430941000); store at 4 °C, avoid contact with air
25% glutaraldehyde (GA) (VWR, Electron Microscopy Sciences, catalog number: 100504-788); store at 4 °C. We have found that this GA can be stored for up to three years without any changes to the end result
40% acrylamide (Sigma-Aldrich, catalog number: A4058); store at 4 °C
2% bis-acrylamide (Sigma-Aldrich, catalog number: M1533); store at 4 °C
Ammonium persulfate powder (APS) (VWR, catalog number: 97064-594), store at room temperature in a desiccator, strong oxidizer
Tetramethylethylenediamine (TEMED) (VWR, catalog number: 97064-684); flammable
125 mm clear acrylic disc (127 mm/5 inch acrylic discs may be found on Amazon, or acrylic sheets may be purchased from McMaster-Carr and laser cut into any size or shape)
Sulfo-SANPAH (Covachem, catalog number: 13414-100); store at 4 °C
HEPES (Sigma-Aldrich, catalog number: H3375-500G)
Protein of interest (e.g., collagen, Corning, catalog number: 354236); store at 4 °C
Hank’s buffered salt solution (HBSS) (ScienCell, catalog number: 0313)
Milli-Q water (or equivalent grade)
Isopropanol (70% solution)
Ethanol (70% solution)
Dimethyl sulfoxide (DMSO) (Sigma-Aldrich, catalog number: 276855)
Ammonium persulfate, 10% w/v solution (see Recipes)
Sulfo-SANPAH, 100 mg/mL solution (see Recipes)
HEPES-buffered saline (HBS) (see Recipes)
Equipment
Vacuum desiccator
Balance/weighing scale
Glass beaker (VWR, catalog number: 13912-284)
Filter forceps (VWR, catalog number: 89259-954)
Scalpel handle #3 (Fine Science Tools, catalog number: 10003-12)
Pipet aid and serological pipets (10, 25, 50 mL)
Set of micropipettes and tips (P10, P200, P1000)
Oven, set at 65 °C
Chemical hood
Biosafety cabinet with vacuum aspirator
Autoclave
UV lamp (e.g., 100-Watt 365 nm UVP lamp)
CO2humidified incubator, set at 37 °C
-20 °C freezer
4 °C refrigerator
Procedure
Creation of polydimethylsiloxane (PDMS) ring molds
Using a balance, weigh Sylgard 184 elastomer base and Sylgard 184 curing agent into a plastic cup at a 9:1 ratio (e.g., 27 g of elastomer base and 3 g of curing agent).
Note: Uncured Sylgard 184 elastomer base is highly viscous.
Mix the PDMS pre-polymer thoroughly with a plastic spoon and pour into plastic 145 mm Petri dishes, making sure the surface is evenly coated. The amount of PDMS pre-polymer can be increased or decreased, but 1–1.5 mm final thickness within the Petri dish is recommended.
Degas Petri dishes in a vacuum chamber to remove air bubbles from pre-polymer mixture.
Cure the PDMS pre-polymer overnight in an oven set at 65 °C.
Note: Make sure the oven surface is leveled for a uniform PDMS thickness. As little as ~1° slope will adversely impact downstream processes.
With gloved hands, remove the cured PDMS discs from the Petri dishes and cut into rings. Using a scalpel, cut out a ring with an outer diameter of 145 mm and an inner diameter of 120 mm to serve as the gasket.
Suggestion: Trace a circle with a diameter of 120 mm on the PDMS disc using a pen or non-solvent resistant marker. The acrylic cover must overlap the PDMS to get a good seal during polymerization.
Clean PDMS rings with 70% isopropanol followed by 70% ethanol. Dry completely.
Seal PDMS rings inside an autoclavable pouch and use a 30 min sterilization cycle with a minimum temperature of 120 °C (see section F for reusability).
Glass dish surface activation
Note: This section should be performed in a chemical fume hood due to hazardous chemical use.
Add enough 0.1 M NaOH to cover the bottom of the 145 mm glass Petri dishes and let sit for 30 min.
Remove excess NaOH and let dishes dry completely.
In a glass beaker, mix APTES into isopropanol to make a 2% solution.
Pour APTES solution into dishes until surfaces are fully covered (20–30 mL/dish) and let sit for 10 min.
Note: APTES is reactive to oxygen. The container from the listed manufacturer has a regenerative rubber seal where the contents are meant to be removed via syringe needle and volumetrically replaced with an inert gas (e.g., N2). APTES loses its reactivity when exposed to molecular oxygen and will therefore become less effective over time. This step extends the shelf life of APTES.
CAUTION: APTES is corrosive; chemical-resistant gloves, lab coat, and goggles should be used.
Following the reaction, remove the APTES solution and add ~30 mL of Milli-Q water to each dish; swirl dishes to mix, let sit for 5 min, and then remove water.
Repeat this step three more times for a total of four exchanges.
For each exchange, dispose in appropriate hazardous waste stream.
Add 20–30 mL of 0.5% glutaraldehyde diluted with Milli-Q water to each of the glass dishes and let sit for 30 min.
Dispose of the glutaraldehyde solution in an appropriate hazardous waste stream and allow dishes to air dry. Proceed to polymerization step (section C) within 24 h of activation for optimal gel attachment.
Polyacrylamide gel polymerization
Note: Parts of this section are recommended to be performed in a biosafety cabinet (BSC) and/or laminar flow hood to minimize contamination. Wipe down all materials with 70% ethanol before placing them in the BSC.
Prepare pre-polymer solutions of acrylamide.
Many polyacrylamide gelrecipesare published in the literature that report the ratios of bis-acrylamide and acrylamide to produce the desired elastic modulus (Aratyn-Schaus et al., 2010;Tse and Engler, 2010; Syed et al., 2015; Charrier et al., 2020).
Using any published reference table, add 40% acrylamide, 2% bis-acrylamide, and Milli-Q water in the desired ratios in a 15 mL conical tube for a total volume of 15 mL of solution per 145 mm glass dish. Optional: Pre-polymer solutions can be made up in larger volume batches, wrapped in parafilm, and stored at 4 °C for 3–4 days. Fresh solutions should be used when possible because even minor evaporation of solution over time can cause noticeable shifts in the storage modulus upon polymerization.
Degas solutions in a vacuum chamber for at least 30 min to remove dissolved oxygen.
In a BSC, use forceps to remove sterile PDMS rings from the autoclave pouch and place them inside the activated glass Petri dishes. Press down firmly on the PDMS ring with the forceps to ensure good contact with the glass.
Spray down acrylic discs with ethanol and dry with cleanroom wipes or compressed air and set inside BSC.
Bring degassed pre-polymer solutions inside BSC. Initiate the reaction by adding ammonium persulfate (1:100 dilution) and TEMED (1:1,000 dilution).
Gently invert tube 2–3 times and pour ~15 mL into the glass Petri dish.
Place the acrylic cover on top of the PDMS ring to create a seal.
Like mounting a coverslip to a slide, grab one side of the acrylic cover with forceps and set onto the PDMS ring at an angle.
Lower the acrylic cover—watch as it makes contacts with the solution and check for any bubbles in the process.
Pipette any excess solution from around the ring, place solution back into the conical tube, and cap the tube. Use the extra solution in the conical tube to gauge when the polyacrylamide has gelled, as it will indicate when the dish is ready.
After the polyacrylamide has fully polymerized (~15 min at room temperature), remove the acrylic cover.
Aspirate unpolymerized solution at the PDMS–acrylic interface.
Note: Unpolymerized solution is expected. The overlap between the PDMS ring and the acrylic cover is designed to prevent uneven gelation at the edges and facilitate removal of the acrylic cover.
Using a P1000 micropipette, add 100% isopropanol into the PDMS–acrylic interface where the unpolymerized solution sat until it penetrates the polyacrylamide–acrylic interface. Gently lift up on the acrylic cover to help the isopropanol enter the gel-cover interface while pipetting.
Once the isopropanol has completely infiltrated the polyacrylamide–acrylic interface, the acrylic cover should pop off very easily.
Wash polyacrylamide gel three times with HBS.
Note: Do not put vacuum aspirator on or directly above the gel as it may get caught in the vacuum. Instead, place the aspirator on the PDMS ring and tip the dish so the fluid falls into the vacuum aspirator.
Polyacrylamide gel dishes can be stored at 4 °C in HBS, sealed with parafilm or plastic wrap.
Matrix protein-polyacrylamide conjugation
Note: This section is recommended to be performed in a BSC with aseptic technique whenever possible, to minimize contamination. The extracellular matrix (ECM) protein of choice (fibronectin, collagen, laminin, etc.) depends on the cell line used and will need to be tested for suitability.
Prepare working solutions of the ECM protein of interest (e.g., collagen at 100 μg/mL) by diluting in HBS.
Remove the storage buffer from the polyacrylamide dishes.
Dilute sulfo-SANPAH stock solution to 0.5 mg/mL in Milli-Q water and pipette directly on the surface of polyacrylamide gels (~5 mL). Sulfo-SANPAH hydrolyzes rapidly in water, so this step needs to be done promptly.
Place the dishes under the UV light for up to 10 min to activate the surface.
Note: Remove the lids to the dishes before placing under UV light.
Sulfo-SANPAH should turn burgundy (dark orange) when it has reacted with the polyacrylamide.
Activation time may change based on UV lamp wavelength, power, and distance of dish from light source. For the lamp listed above, a distance of three inches from the light source for 10 min is sufficient.
Wash the polyacrylamide dishes three times with HBS to remove excess sulfo-SANPAH.
Add the ECM protein solution directly on top of the polyacrylamide and place the dishes in a cell culture incubator at 37 °C overnight.
Roughly 10 mL of solution is sufficient for a uniform conjugation.
We found that an overnight incubation worked well, but less time (1–2 h) may be sufficient.
After incubating with the protein solution, wash once with HBS and replace with fresh HBS.
Soak the gels in HBS overnight (or 2–3 h) at 37 °C.
Repeat this step with one more exchange of fresh HBS to remove excess unconjugated sulfo-SANPAH and/or ECM protein if necessary.
The gel should turn to a very light transparent orange.
Collagen can be visualized under a light microscope equipped with a 10× objective, which can be used to check for any non-uniformity.
Dishes can be stored at 4 °C in HBS sealed with parafilm or plastic wrap for weeks.
Cell culture
Note: This section should be performed in a BSC with aseptic technique whenever possible, to minimize contamination.
Remove HBS and incubate dishes with fresh cell culture media for 1 h at 37 °C prior to seeding cells.
Plate cells by dripping the cell suspension over the entire surface and allow to adhere for ~1 h.
Add 25–30 mL of media once cells have adhered to the surface.
When cells are ready to be passaged, remove the cell media, add warm HBSS or serum-free media to the dishes, and let sit for 15–30 min in a cell culture incubator.
Note: This will wash the cells in preparation for trypsinization.
If cell media contains a high concentration of serum (e.g., 10% fetal bovine serum) or polyacrylamide gels are softer (< 10 kPa), more than one exchange may be necessary to remove excess protein within the gels.
Serum proteins must be adequately removed to prevent inhibition of the trypsin.
If phenol is present in cell media, the polyacrylamide gels should change from a pink color to almost clear, indicating the cells are ready to be trypsinized.
Remove HBSS or serum-free media and proceed with routine passaging.
Cleaning and reusing of the glass dish
With gloved hands, remove PDMS ring, clean with warm, soapy water, and sterilize in the autoclave. PDMS rings can be placed inside a beaker of isopropanol and/or detergent solution before sterilization to remove absorbed material.
Remove polyacrylamide from the dish. Polyacrylamide can be removed by gloved hand or with forceps.
Bleach glass dish for at least 15 min, rinse with copious water, and clean with warm soapy water.
Sterilize dishes in autoclave. Dishes are ready to be re-used (start at section B: Glass dish surface activation).
Data analysis
Cell viability and cell debris measurements were performed in technical and experimental triplicates using an Agilent NovoCyte flow cytometer. An ordinary one-way ANOVA was performed on cell viability data and Brown-Forsythe and Welch’s ANOVA were performed on cell debris data.
Recipes
Ammonium persulfate, 10% w/v solution
Dissolve in deionized water at a concentration of 10% w/v. Ammonium persulfate should crackle as it dissolves in water.
Aliquot and store at -20 °C for up to six months.
Sulfo-SANPAH, 100 mg/mL solution
Dissolve sulfo-SANPAH in DMSO at 100 mg/mL.
Aliquot in 25 μL volumes in microcentrifuge tubes and freeze at -80 °C for up to one year.
HEPES buffered saline (HBS)
Dissolve 50 mM HEPES and 0.9% w/v NaCl in Milli-Q water.
Bring to pH 8.0 with NaOH.
Sterile filter solution using 0.2 μm PES bottle top filter.
Acknowledgments
This work was funded by the National Institutes of Health (R01GM26643, R01HL133163, R01HL145147, U19AI158930). The protocol was adapted from Millar-Haskell et al. (2022). The authors would like to acknowledge John Sperduto and Bryan Ferrick for their assistance in acquiring materials.
Competing interests
The authors declare no competing interests.
Ethics
No animal or human subjects were used in this work.
References
- Aratyn-Schaus, Y., Oakes, P. W., Stricker, J., Winter, S. P. and Gardel, M. L. (2010). Preparation of Complaint Matrices for Quantifying Cellular Contraction. J. Vis. Exp.: e3791/2173.
- Chandler, E. M., Berglund, C. M., Lee, J. S., Polacheck, W. J., Gleghorn, J. P., Kirby, B. J. and Fischbach, C. (2011). Stiffness of photocrosslinked RGD-alginate gels regulates adipose progenitor cell behavior. Biotechnol. Bioeng. 108(7): 1683–1692.
- Charrier, E. E., Pogoda, K., Li, R., Park, C. Y., Fredberg, J. J. and Janmey, P. A. (2020). A novel method to make viscoelastic polyacrylamide gels for cell culture and traction force microscopy. APL Bioeng. 4(3): 036104.
- Chen, W. L. K. and Simmons, C. A. (2011). Lessons from (patho)physiological tissue stiffness and their implications for drug screening, drug delivery and regenerative medicine. Adv. Drug Delivery Rev. 63: 269–276.
- Engler, A. J., Sen, S., Sweeney, H. L. and Discher, D. E. (2006). Matrix Elasticity Directs Stem Cell Lineage Specification. Cell 126(4): 677–689.
- Gleghorn, J. P., Manivannan, S. and Nelson, C. M. (2013). Quantitative approaches to uncover physical mechanisms of tissue morphogenesis. Curr. Opin. Biotechnol. 24(5): 954–961.
- Kumai, J., Sasagawa, S., Horie, M. and Yui, Y. (2021). A Novel Method for Polyacrylamide Gel Preparation Using N-hydroxysuccinimide-acrylamide Ester to Study Cell-Extracellular Matrix Mechanical Interactions. Front. Mater. 8: e637278.
- Lachowski, D., Cortes, E., Pink, D., Chronopoulos, A., Karim, S. A., P. Morton, J. and del Río Hernández, A. E. (2017). Substrate Rigidity Controls Activation and Durotaxis in Pancreatic Stellate Cells. Sci. Rep. 7(1): e1038/s41598-017-02689-x.
- Mih, J. D., Sharif, A. S., Liu, F., Marinkovic, A., Symer, M. M. and Tschumperlin, D. J. (2011). A Multiwell Platform for Studying Stiffness-Dependent Cell Biology. PLoS One 6(5): e19929.
- Millar-Haskell, C. S., Dang, A. M. and Gleghorn, J. P. (2019). Coupling synthetic biology and programmable materials to construct complex tissue ecosystems. MRS Commun. 9(2): 421–432.
- Millar‐Haskell, C. S., Sperduto, J. L., Slater, J. H., Thorpe, C. and Gleghorn, J. P. (2022a). Secretion of the disulphide bond generating catalyst QSOX1 from pancreatic tumour cells into the extracellular matrix: Association with extracellular vesicles and matrix proteins. J. Extracell. Biol. 1(7): e48.
- Millar-Haskell, C. S., Thorpe, C. and Gleghorn, J. P. (2022b). Matrix mechanics, not hypoxia, modulate quiescin sulfhydryl oxidase 1 (QSOX1) in pancreatic tumor cells. bioRxiv: e512796.
- Morgan, J. T., Shirazi, J., Comber, E. M., Eschenburg, C. and Gleghorn, J. P. (2019). Fabrication of centimeter-scale and geometrically arbitrary vascular networks using in vitro self-assembly. Biomaterials 189: 37–47.
- Nelson, C. M. and Gleghorn, J. P. (2012). Sculpting Organs: Mechanical Regulation of Tissue Development. Annu. Rev. Biomed. Eng. 14(1): 129–154.
- Stanton, A. E., Tong, X. and Yang, F. (2019). Extracellular matrix type modulates mechanotransduction of stem cells. Acta Biomater. 96: 310–320.
- Syed, S., Karadaghy, A. and Zustiak, S. (2015). Simple Polyacrylamide-based Multiwell Stiffness Assay for the Study of Stiffness-dependent Cell Responses. J. Vis. Exp.: e3791/52643.
- Trompeter, N., Farino, C. J., Griffin, M., Skinner, R., Banda, O. A., Gleghorn, J. P., Slater, J. H. and Duncan, R. L. (2021). Extracellular Matrix Stiffness Alters TRPV4 Regulation in Chondrocytes. bioRxiv: e460172.
- Tse, J. R. and Engler, A. J. (2010). Preparation of Hydrogel Substrates with Tunable Mechanical Properties. Curr. Protoc. Cell Biol. 47(1): ecb1016s47.
Article Information
Publication history
Available online: Sep 5, 2023
Published: Sep 5, 2023
Copyright
© 2023 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
How to cite
Millar-Haskell, C. S. and Gleghorn, J. P. (2023). A Large-format Polyacrylamide Gel with Controllable Matrix Mechanics for Mammalian Cell Culture and Conditioned Media Production. Bio-protocol 13(17): e4807. DOI: 10.21769/BioProtoc.4807.
Category
Cell Biology > Cell isolation and culture > 3D cell culture
Biological Engineering > Biomedical engineering
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Tips for asking effective questions
+ Description
Write a detailed description. Include all information that will help others answer your question including experimental processes, conditions, and relevant images.
Share
Bluesky
X
Copy link







