- Submit a Protocol
- Receive Our Alerts
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Mouse Corneal Epithelial and Stromal Cell Isolation and Culture
Published: Vol 13, Iss 19, Oct 5, 2023 DOI: 10.21769/BioProtoc.4829 Views: 397
Reviewed by: Pilar Villacampa AlcubierreMarina Sánchez PetidierAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Human Schwann Cells in vitro I. Nerve Tissue Processing, Pre-degeneration, Isolation, and Culturing of Primary Cells
Gabriela I. Aparicio and Paula V. Monje
Nov 20, 2023 512 Views
Isolation of Embryonic Cardiomyocytes and Cell Proliferation Assay Using Genetically Engineered Reporter Mouse Model
Maren Beall [...] Jihyun Jang
Sep 5, 2023 475 Views
Isolation and Culture of Neural Stem/Progenitor Cells from the Hippocampal Dentate Gyrus of Young Adult and Aged Rats
Mina Afhami [...] Koorosh Shahpasand
Oct 5, 2023 282 Views
Abstract
Corneal epithelium and stroma are the major cellular structures for ocular protection and vision accuracy; they play important roles in corneal wound healing and inflammation under pathological conditions. Unlike human, murine corneal and stromal fibroblast cells are difficult to isolate for cell culture. In our laboratory, we successfully used an ex vivo culture procedure and an enzymatic procedure to isolate, purify, and culture mouse corneal epithelial and stromal fibroblast cells.
Key features
• Primary cell culture models of a disease are critical for cellular and molecular mechanism studies.
• Corneal tissues with the limbus contain stem cells to generate both epithelial and stromal cells.
• An ex vivo corneal culture provides a constant generation of primary corneal cells for multiple passages.
• The isolated cells are validated by the corneal epithelial cell markers Krt12 and Cdh1 and the stromal fibroblast marker Vim.
Keywords: Mouse corneaBackground
The cornea is a transparent avascular tissue; it acts as a barrier to protect the eye and has a 2/3 focal power to project an image onto the retina. Mouse cornea consists of three cellular layers: the epithelium, the stroma, and the endothelium (Figure 1). The epithelium consists of a differentiated superficial squamous cell, a transit wing cell, and a proliferative basal cell type, and is the outmost ocular tissue that contains 80% corneal cells. The innermost corneal tissue is the endothelium, consisting of a single unrenewable cell sheet. The epithelium layer and the endothelium layer are separated from the stroma by the Bowman’s membrane and the Descemet’s membrane, respectively. The stroma is the thickest and toughest part of the cornea in between the epithelium and the endothelium. Although the stroma contains only approximately 20% of the corneal cells, it occupies two thirds of the corneal space. Both epithelial and stromal cells are embedded in a tough collagen-rich extracellular matrix (ECM) [1]. As the epithelial cells can be regenerated by a group of stem cells, also known as limbal epithelial stem cells, at the limbus (the border area between the cornea and the sclera) for cellular homeostasis and wound repair, it is relatively easy to isolate and culture them [2]. However, the corneal stromal fibroblast (keratocyte) may be regenerated at a relatively slower pace; its regeneration is supposed to be either local and/or come from the progenitor at the limbus region. Isolation and culture of corneal cells, i.e., epithelial cells and stromal keratocytes, are critical for the study of corneal diseases, particularly wound healing [3] and neovascularization [4], as well as their underlying molecular mechanisms [5]. Human corneal primary epithelial and stromal cells and murine primary corneal epithelial cells are commercially available; however, no murine primary stromal fibroblast cells can be purchased. It is difficult to isolate and culture murine primary corneal cells, particularly murine keratocytes. A protocol to isolate and culture murine keratocytes is available for newborn pups, where the separation of the stroma from the epithelium is very difficult and the resultant cell identification was not verified by a specific marker [6]. We have developed a relatively simple and reliable method to isolate both murine corneal epithelial cells and keratocytes, and culture them in vitro for multiple passages.

Figure 1. Mouse corneal structure and cell types
Materials and reagents
Biological materials
Animals: 4–8-week-old B6 mice (The Jackson Laboratory, catalog number: 000664)
Consumable materials
Cell culture plate: 12-well cell culture plates (Thermo Fisher Scientific, catalog number: 150628)
Eppendorf tubes (Thermo Fisher Scientific, catalog number: 3451)
Tipone® pipette tips (USA Scientific, catalog number: 1126-7810)
Pipettes (Corning, Falcon®, catalog number: 357543)
Cryopreservation vials (Corning, catalog number: 430659)
Reagents
Ethanol
Phosphate-buffered saline (PBS) (Sigma-Aldrich, catalog number: P4417-100TAB)
Penicillin-streptomycin (5,000 U/mL) (Thermo Fisher Scientific, catalog number: 15070063)
Dulbecco’s modified Eagle’s medium (DMEM, high glucose) (Thermo Fisher Scientific, catalog number: 11965)
Fetal bovine serum (FBS) (GE Healthcare, HyClone, catalog number: SH30071.03)
Gelatin (Sigma-Aldrich, catalog number: G1890-100G)
Collagenase A (Millipore Sigma, catalog number: 10103578001)
Trypsin ethylenediaminetetraacetic acid (EDTA) solution (Mediatech, Cellgro®, catalog number: 25-053-CI)
Common epithelial marker E-cadherin (Cdh1) (BD Transduction Lab, catalog number: 610181)
Specific epithelial marker Keratin 12 (Krt12) (Abclonal, catalog number: A9642)
Fibroblast marker vimentin (Sigma, catalog number: 17-10195)
100× penicillin-streptomycin solution (Thermo Fisher Scientific, catalog number: 15140122)
Corneal epithelial cell basal medium (ATCC, catalog number: PCS-700-030)
Corneal epithelial cell growth kit (ATCC, catalog number: PCS-700-040)
Solutions
Corneal epithelial cell culture medium (see Recipes)
Corneal stromal fibroblast culture medium (see Recipes)
0.1% gelatin solution (see Recipes)
Recipes
Corneal epithelial cell culture medium
Prepare the complete corneal epithelial growth medium according to the manufacturer’s instructions. The final concentration for each component in ATCC complete corneal epithelial growth medium is as follows:
5 μg/mL Apo-transferrin
1.0 μM epinephrine
0.4% extract P
100 ng/mL hydrocortisone
6 mM L-glutamine
5 μg/mL recombinant human insulin
CE Growth Factor: proprietary formulation
Corneal stromal fibroblast culture medium
450 mL of DMEM
50 mL of FBS
5 mL of penicillin/streptomycin (5,000 U/mL)
0.1% gelatin solution
0.1 g gelatin powder
100 mL of PBS
Autoclave and store at 4 °C for three months.
Equipment
Mcpherson-Vannas curved iris scissors (Storz Ophthalmic Instruments, catalog number: E3347)
Castroviejo suturing forceps 0.12 mm (Storz Ophthalmic Instruments, catalog number: E1796)
Martinez double ended corneal dissector (Storz Ophthalmic Instruments, catalog number: E3001)
Algerbrush II (Katena Products, Inc., Denville, NJ)
Cell culture incubator (Thermo Fisher Scientific, Thermo Scientific, model: 3250)
Water bath (VWR, model: 1545)
Pipettes (Eppendorf)
Pipet-aid (Drummond)
Benchtop centrifuge (Beckman Coulter, model: Allegra® X-15R)
Lab rotating shaker (Barnstead Thermolyne LabQuake, model: 4152110)
Inverted microscope (Nikon, Eclipse TS100)
Confocal microscope (Nikon, Eclipse Ti)
40 μm nylon net filter (Fisher Scientific, Leicestershire, UK)
Procedure
Corneal epithelial cell isolation and culture
All materials used in this procedure must be sterile or autoclaved to prevent contamination (Figure 2).

Figure 2. Surgery tools used in the procedure must be autoclavedEuthanize mice by carbon dioxide followed by cervical dislocation to confirm death.
Enucleate both eyeballs using tweezers and place them in 1% penicillin-streptomycin PBS (Figure 3A).
Carefully trim off the cornea of full thickness from the eyeball with a pair of surgery scissors under a microscope (Figure 3B).
Thereafter, cut the entire cornea with the limbus quarterly into a butterfly tie-like shape using scissors (Figure 3C) and place it in 1% penicillin-streptomycin PBS.
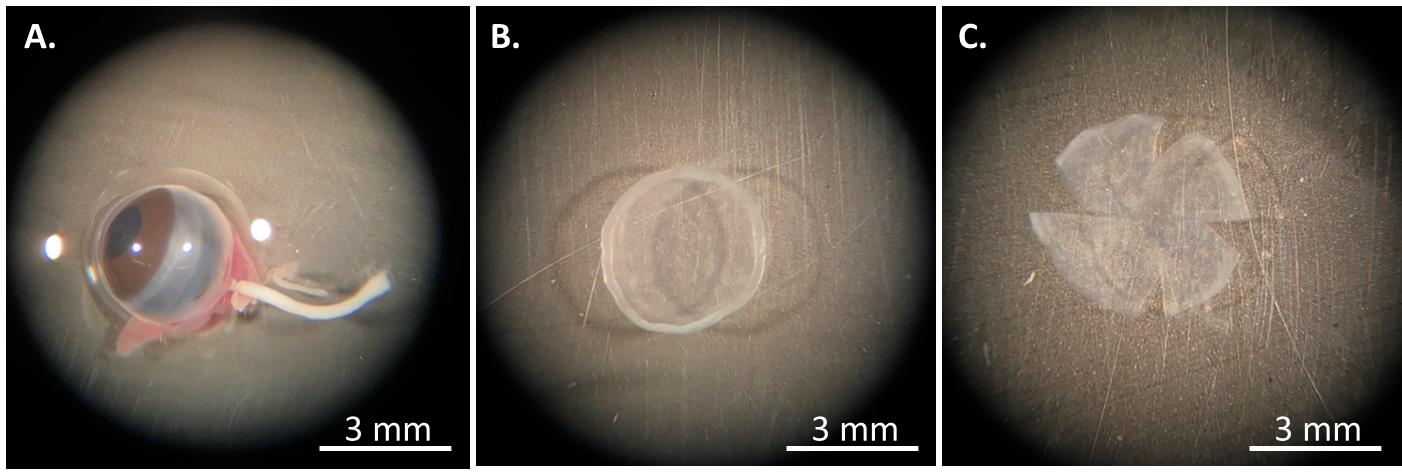
Figure 3. The procedure of mouse corneal isolation. (A) The eyeball is enucleated and placed in PBS buffer. (B) The entire cornea with the limbus is cut off, and (C) quarterly divided into a butterfly tie-like shape.To coat a 12-well culture plate, prepare 0.1% gelatin solution (Recipe 3) and sterilize it through filtration. Add 100 μL of this coating solution to each well and leave at room temperature for 10 min.
Aspirate the coating solution from the plate and place 2–4 corneas with the epithelium side down on each well in order to have enough epithelial cells moving out of the tissues.
Gently press the tissue piece with tweezers to make the tissue fully contact the surface of the plate; add 100 μL of corneal epithelial culture medium (Recipe 1) to cover the entire corneal tissue.
Incubate the plate with cultured corneas at 37 °C under 100% humidity and 5% CO2.
On the second day, very slowly add 500 μL of the corneal epithelial culture medium along the well wall to prevent the tissue from floating.
When most epithelial cells move out of the epithelial tissue onto the plate, approximately at day 7 after seeding (Figure 4A–4D), remove the corneal tissue with tweezers and add 1 mL of fresh corneal epithelial culture medium.
Replace the old medium with 1.5 mL of new medium every 3–4 days until cell confluence. Reuse the ex vivo corneal tissue at least five times.
After removing the culture medium, detach the confluent murine corneal epithelial cells from the plate and from each other by incubating them with 0.3 mL of 0.15% trypsin EDTA solution at 37 °C for 3 min.
Split the cells once at 1:2 ratio into two separate wells with 3 mL of the corneal stromal fibroblast culture medium (Recipe 2) overnight to inactivate the trypsin and help cells adhere to the plate.
On the next day, replace the corneal stromal fibroblast culture medium with 1.5 mL of corneal epithelial culture medium.
The corneal epithelial cells are confirmed by their small, compact, round, or elliptical morphology (Figure 4E) and immunostaining (Figure 4F–4G) with the corneal epithelial marker keratin 12 (Krt12) and the common epithelial marker E-cadherin (Cdh1).
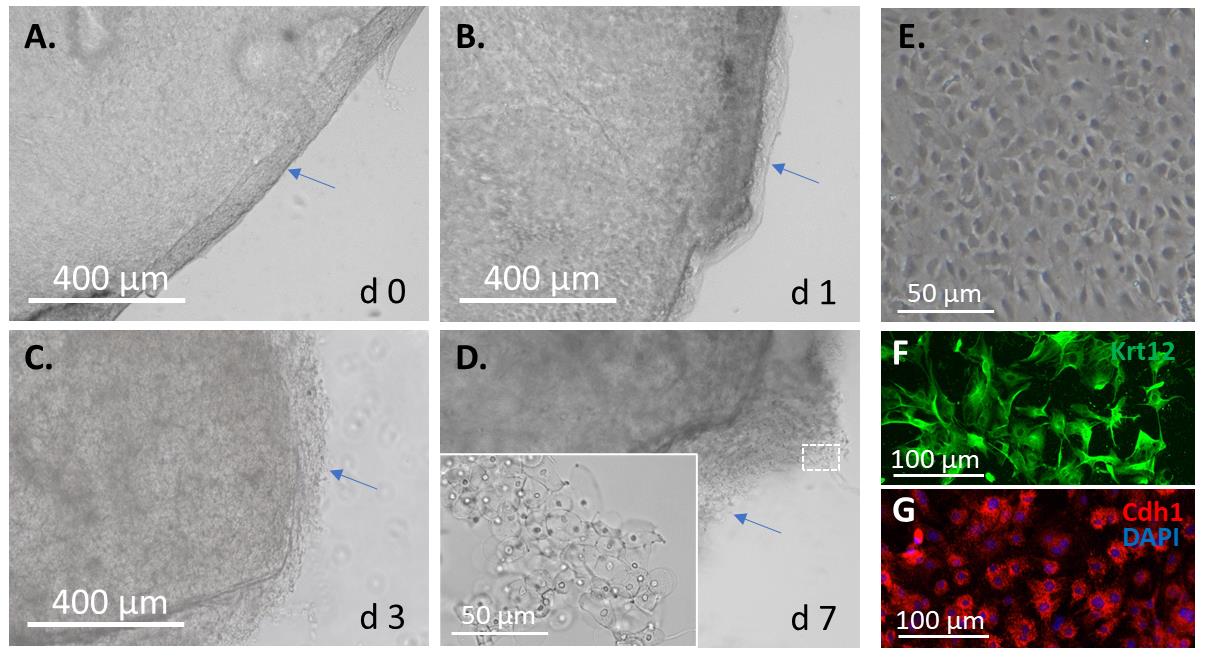
Figure 4. Mouse corneal epithelial cell isolation and culture. The isolated mouse cornea is placed upside down on a cell culture plate coated with 0.1% gelatin for (A) 0, (B) 1, (C) 3, and (D) 7 days. (E) The monolayer-cultured corneal epithelial cells and their immunofluorescence with (F) the corneal epithelial marker keratin 12 (Krt12) and (G) the common epithelial maker E-cadherin (Cdh1).
Corneal stroma cell isolation and culture
Euthanize mice by carbon dioxide followed by cervical dislocation to confirm death.
Remove the epithelial layer down to the basement membrane by mechanical scraping using an Algerbrush II and a cotton swab (Video 1 shows the head tip of Algerbrush II scrapping corneal epithelial cells into a cell debris mass; after the entire epithelium area is debrided, the cell debris mass is cleaned by a cotton swab).
Video 1. Debridement of the corneal epithelium using Algerbrush IIEnucleate both eyeballs and place them in 1% penicillin-streptomycin PBS.
Carefully trim off the cornea of full thickness from the eyeball with a pair of surgery scissors under a microscope (Figure 3B).
Thereafter, cut the entire cornea with the limbus quarterly into a butterfly tie-like shape (Figure 3C) and place it in 1% penicillin-streptomycin PBS.
Remove the endothelial layer using Martinez double ended corneal dissector (Video 2 shows that the stroma of cornea noticeably becomes clearer after the removal of the endothelium) and cut the remainder of the stromal tissue into small pieces using a pair of surgery scissors and a razor blade.
Video 2. Removal of the corneal endothelium using Martinez dissectorFurther digest each minced corneal tissue in 0.5 mL of 0.1 mg/mL collagenase A in an Eppendorf tube at 37 °C for 1–2 h. Vortex the tube every 10 min until the digestion solution appears cloudy, a sign of thorough tissue digestion.
Filtrate the digested corneal tissue with a 40 μm nylon net filter to remove bigger debris and to collect the flowthrough stromal cells in a 15 mL tube.
Add the corneal stromal fibroblast culture medium to the flowthrough solution and centrifuge at 1,000× g for 5 min.
Decant the supernatant, resuspend the cell pellet in 3 mL of the corneal stromal fibroblast culture medium (Recipe 2), and place it in one well of a 6-well culture plate.
Refresh the medium every 3–4 days until cell confluence.
After removal of the culture medium, detach the confluent murine corneal stromal fibroblast cells from the plate and from each other by incubating with 0.3 mL of 0.15% trypsin solution at room temperature for 3 min.
Split the cells once at 1:2 ratio into two separate wells with 3 mL of the corneal stromal fibroblast culture medium to inactivate the trypsin and help cells adhere to the plate.
The corneal fibroblasts (keratocytes) are confirmed by their morphology (Figure 5A–5C) and immunostaining (Figure 5D) with the fibroblast marker vimentin.
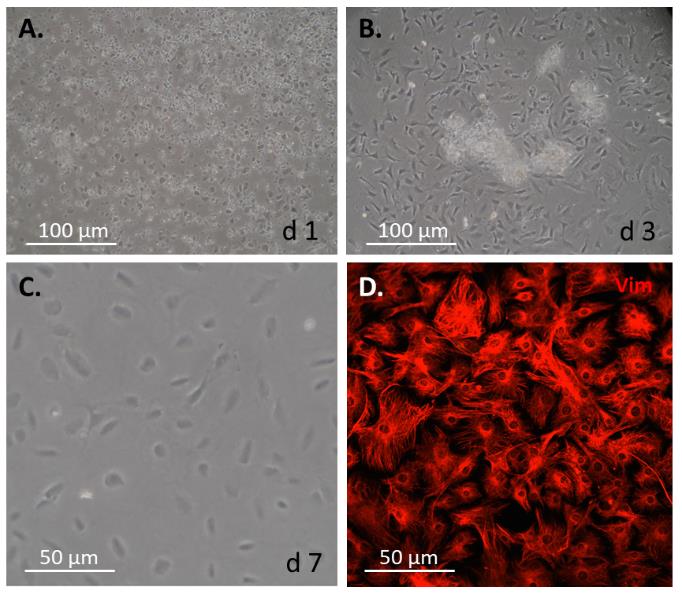
Figure 5. Mouse corneal stromal fibroblast cell isolation and culture. The epithelium and the endothelium are first removed mechanically from the isolated mouse cornea; the remaining stroma is minced and then further digested with collagenase type A. Upon a passage through a nylon net filter, the isolated cells are cultured in a plate coated with 0.1% gelatin for (A) 1, (B) 3, and (C) 7 days. (D) Monolayer-cultured mouse corneal stromal cells stained with the fibroblast cell marker vimentin (Vim).
Validation of protocol
We used this protocol to isolate and culture mouse corneal epithelial and stromal cells for our previous studies and had reliable results [3].
General notes and troubleshooting
These protocols for murine corneal epithelial and stromal fibroblast cell isolation and culture are relatively simple and easy for research laboratories in need, though their isolation yield and proliferation rates in culture are still low. We do not know why it is so technically difficult. Compared to human, mouse life span is very short; we believe that the renewability of mouse primary ocular cells, such as RPE and Müller cells to our best knowledge, is probably not as strong as human primary ocular cells.
Acknowledgments
This protocol was recently adopted in the laboratory [4]. The work was supported by National Institute of General Medical Sciences (P20GM103453 to Y.L.), University of Louisville School of Medicine (E0819 to Y.L.), James Graham Brown Cancer Center of University of Louisville Directed Gift Pilot Project Program (G1779 to Y.L.), and the National Natural Science Foundation of China (82171032 L.Z.), the Natural Science Foundation of Liaoling Province (201800209, 201602210, and 20180550976 to L.Z.).
Competing interests
The authors declare no competing interests.
Ethical considerations
Use of animals in this protocol was conducted according to the policies and guidelines set forth by the Institutional Animal Care and Use Committee (IACUC) and approved by the University of Louisville, Kentucky, USA.
References
- Espana, E. M. and Birk, D. E. (2020). Composition, structure and function of the corneal stroma. Exp. Eye Res. 198: 108137. doi: 10.1016/j.exer.2020.108137
- Kobayashi, T., Yoshioka, R., Shiraishi, A. and Ohashi, Y. (2009). New technique for culturing corneal epithelial cells of normal mice. Mol. Vis. 15: 1589–1593. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2728571/
- Zhang, Y., Do, K. K., Wang, F., Lu, X., Liu, J. Y., Li, C., Ceresa, B. P., Zhang, L., Dean, D. C., Liu, Y., et al. (2023). Zeb1 facilitates corneal epithelial wound healing by maintaining corneal epithelial cell viability and mobility. Commun. Biol. 6(1): e1038/s42003-023-04831-0. doi: 10.1038/s42003-023-04831-0
- Jin, L., Zhang, Y., Liang, W., Lu, X., Piri, N., Wang, W., Kaplan, H. J., Dean, D. C., Zhang, L., Liu, Y., et al. (2020). Zeb1 promotes corneal neovascularization by regulation of vascular endothelial cell proliferation. Commun. Biol. 3(1): e1038/s42003-020-1069-z. doi: 10.1038/s42003-020-1069-z
- Liang, W., Zhang, Y., Zhou, L., Lu, X., Finn, M. E., Wang, W., Shao, H., Dean, D. C., Zhang, L., Liu, Y., et al. (2022). Zeb1 regulation of wound-healing-induced inflammation in alkali-damaged corneas. iScience 25(4): 104038. doi: 10.1016/j.isci.2022.104038
- Zhang, Y., Wang, Y. C., Yuka, O., Zhang, L. and Liu, C. Y. (2016). Mouse Corneal Stroma Fibroblast Primary Cell Culture. Bio Protoc 6(19): e1960. doi: 10.21769/bioprotoc.1960
Article Information
Publication history
Published: Oct 5, 2023
Copyright
© 2023 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
How to cite
Zhang, Y., Zhang, L. and Liu, Y. (2023). Mouse Corneal Epithelial and Stromal Cell Isolation and Culture. Bio-protocol 13(19): e4829. DOI: 10.21769/BioProtoc.4829.
Category
Cell Biology > Cell isolation and culture > Monolayer culture
Biological Engineering > Biomedical engineering
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Tips for asking effective questions
+ Description
Write a detailed description. Include all information that will help others answer your question including experimental processes, conditions, and relevant images.
Share
Bluesky
X
Copy link








