- Submit a Protocol
- Receive Our Alerts
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
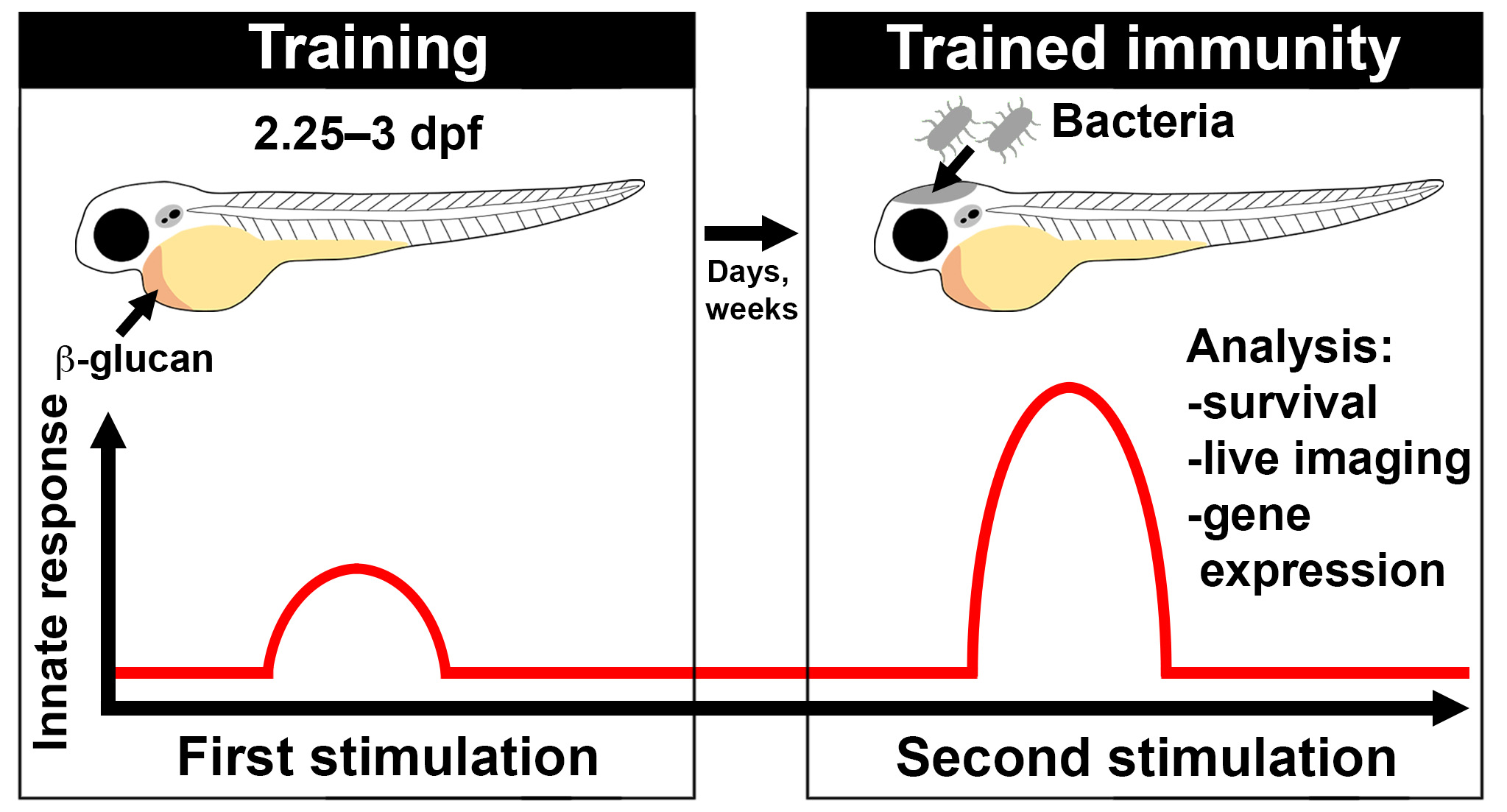
Microinjection of β-glucan Into Larval Zebrafish (Danio rerio) for the Assessment of a Trained-Like Immunity Phenotype
Published: Vol 13, Iss 23, Dec 5, 2023 DOI: 10.21769/BioProtoc.4888 Views: 71
Reviewed by: Alba BlesaWei FanAlberto Rissone
Abstract
The innate immune system can remember previous inflammatory insults, enabling long-term heightened responsiveness to secondary immune challenges in a process termed “trained immunity.” Trained innate immune cells undergo metabolic and epigenetic remodelling and, upon a secondary challenge, provide enhanced protection with therapeutic potential. Trained immunity has largely been studied in innate immune cells in vitro or following ex vivo re-stimulation where the primary insult is typically injected into a mouse, adult zebrafish, or human. While highly informative, there is an opportunity to investigate trained immunity entirely in vivo within an unperturbed, intact whole organism. The exclusively innate immune response of larval zebrafish offers an attractive system to model trained immunity. Larval zebrafish have a functional innate immune system by 2 days post fertilisation (dpf) and are amenable to high-resolution, high-throughput analysis. This, combined with their optical transparency, conserved antibacterial responses, and availability of transgenic reporter lines, makes them an attractive alternative model to study trained immunity in vivo. We have devised a protocol where β-glucan (one of the most widely used experimental triggers of trained immunity) is systemically delivered into larval zebrafish using microinjection to stimulate a trained-like phenotype. Following stimulation, larvae are assessed for changes in gene expression, which indicate the stimulatory effect of β-glucan. This protocol describes a robust delivery method of one of the gold standard stimulators of trained immunity into a model organism that is highly amenable to several non-invasive downstream analyses.
Key features
• This protocol outlines the delivery of one of the most common experimental stimulators of trained immunity into larval zebrafish.
• The protocol enables the assessment of a trained-like phenotype in vivo.
• This protocol can be applied to transgenic or mutant zebrafish lines to investigate cells or genes of interest in response to β-glucan stimulation.
Graphical overview
Key features
Keywords: Larval zebrafish
Background
Trained immunity describes the phenomenon of a long-lived shift in responsiveness of innate immunity following an earlier immune challenge, whereby the innate immune system remembers previous infectious encounters. Training induces metabolic and epigenetic changes within innate immune cells (including macrophages, neutrophils, and natural killer cells) as well as non-immune cells (such as fibroblasts, intestinal stromal cells, and epithelial stem cells) that typically result in a heightened response to a secondary challenge (Hamada et al., 2019; Netea et al., 2020). Trained immunity has been induced by a range of stimuli including vaccines (Kleinnijenhuis et al., 2012; Arts et al., 2018; Kaufmann et al., 2018), metabolites (Arts et al., 2016; Ferreira et al., 2023), and fungal polysaccharides such as β-glucan (Arts et al., 2016; Mitroulis et al., 2018; Kalafati et al., 2020). Trained immunity has been largely studied in vitro or following ex vivo re-stimulation of cells extracted from trained mice, adult zebrafish, or humans (Kleinnijenhuis et al., 2012; Kaufmann et al., 2018; Rodríguez et al., 2009; Megías et al., 2016; Arts et al., 2018). While highly informative, in vitro and ex vivo re-stimulation of cells may not fully mimic the in vivo response (Silliman and Wang, 2006). A vertebrate model where the innate immune response can be analysed entirely in vivo, such as larval zebrafish, offers an attractive additional model to investigate trained immunity.
Larval zebrafish possess a functional innate immune system by 2 days post fertilisation (dpf) but only develop a functionally mature adaptive immune system by 4–6 weeks post fertilisation (Lam et al., 2004). This chronological separation enables the study of the innate immune response in isolation during larval stages. Additionally, larval zebrafish offer powerful downstream analysis techniques including single-cell resolution microscopy using transgenic reporter lines to directly observe innate immune cell (macrophage and neutrophil) responses. β-glucan is a conventional training stimulus with well-characterised immune-potentiating effects that have been demonstrated in both adult and larval zebrafish (Rodríguez et al., 2009; Medina-Gali et al., 2018; Darroch et al., 2022). We recently showed that systemic injection of β-glucan induces a trained-like phenotype in larval zebrafish that elevates survival to subsequent infectious challenges and increases the recruitment of neutrophils to infection (Darroch et al., 2022). In this protocol, we outline a method for microinjecting β-glucan into the circulation of larval zebrafish. A soluble form of β-glucan that does not trigger the activation of Dectin-1 (the main pattern recognition receptor involved in the detection of fungal polysaccharides) or downstream NF-kB signalling is used as a control (Roesner et al., 2019). Using this protocol, the expression of genes involved in the NF-kB pathway (ikbaa, nfkb1, nfkb2) as well as genes expressing inflammatory cytokines (il1b, tnfa, cxcl8a, and il10) are assessed following β-glucan stimulation (Rogers et al., 2005; Inoue and Shinohara, 2014; Megías et al., 2016). We found that two of these genes, il10 and tnfa, are significantly upregulated in larval zebrafish at 1 day post stimulation (Darroch et al., 2022). This protocol can facilitate the investigation of downstream β-glucan-induced changes in innate immune responses using different infection models and the large array of available zebrafish transgenic reporter lines.
Materials and reagents
Biological materials
Zebrafish (Danio rerio)
Reagents
TRIzolTM reagent (Invitrogen, catalog number: 15596026)
Chloroform (Sigma-Aldrich, catalog number: C2432)
Isopropanol (2-propanol) (Sigma-Aldrich, catalog number: I9516)
Ethanol absolute (Sigma-Aldrich, catalog number: 1.07017)
β-glucan peptide from Trametes versicolor (Invivogen, catalog code: tlrl-bgp)
Whole glucan particles (soluble, β-glucan control) (Invivogen, catalog number: tlrl-wgps)
Methylcellulose (Sigma-Aldrich, catalog number: M0512)
Phenol red (Sigma-Aldrich, catalog number: P3532)
Tricaine (Ethyl 3-aminobenzoate methanesulfonate salt) (Sigma-Aldrich, catalog number: A5040)
Phosphate buffered saline (PBS), pH 7.4 (Gibco, catalog number: 10010023)
Ultrapure water (Invitrogen, catalog number: 10977015)
MilliQ water
Mineral oil (Sigma-Aldrich, catalog number: M5904)
1-phenyl-2-thiourea (PTU) (Sigma-Aldrich, catalog number: P7629)
iScriptTM cDNA Synthesis kit (Bio-Rad, catalog number: 1708891)
PerfeCTa SYBR Green FastMix with ROX (Quanta Biosciences, catalog number: 95055-500)
1 M Tris (pH 9) (Thermo Scientific, catalog number: J62085-K2)
KCl (Sigma-Aldrich, catalog number: P9541)
CaCl2 (Sigma-Aldrich, catalog number: C4901)
MgSO4 (Sigma-Aldrich, catalog number: 208094)
NaCl (Sigma-Aldrich, catalog number: S3014)
RT qPCR primers—diluted 10 μM in ultrapure water (IDT) (Table 1)
Table 1. RT qPCR primer sequences
Gene Forward (5′ – 3′) Reverse (5′ – 3′) ef1a TGCCTTCGTCCCAATTTCAG TACCCTCCTTGCGCTCAATC il6 AGACGAGCAGTTTGAGAGAGATT GTTTGAGGAGAGGAGTGCTGAT cxcl8a CTGCATTGAAACAGAAAGCC CTTGACTTCACAGGTGATCC tnfa CCAGGGCAATCAACAAGATG TGGTCATCTCTCCAGTCTAAGG il10 TTAAAGCACTCCACAACCC AGTACCTCTTGCATTTCACC il1b ATCAAACCCCAATCCACAGAGT GGCACTGAATCCACCACGTT ikbaa CCTGCGTTCCATTCTCACCT GGCCACTACACTGCTCCTTT nfkb1 CGCAAGTCCTACCCACAAGT ACCAGACTGTGAGCGTGAAG nfkb2 CATATGTCCCACACAATCAAGAC AGCCACCATAATGATCTGGAA
Solutions
E3 (embryo medium) (see Recipes)
Tricaine (see Recipes)
75% Ethanol (see Recipes)
β-glucan stock (see Recipes)
β-glucan control stock (see Recipes)
β-glucan injection mix (see Recipes)
0.5% Phenol red (see Recipes)
3% Methylcellulose (see Recipes)
iScript reaction mix (see Recipes)
PerfeCTa SYBR Green FastMix with ROX mix (see Recipes)
Recipes
E3 (embryo medium) (1×)
Reagent Final concentration Quantity CaCl2 0.33 mM 36.6 mg MgSO4 0.33 mM 39.7 mg KCl 0.17 mM 12.7 mg NaCl 5 mM 292.2 mg MilliQ water n/a Up to 1 L Total n/a 1 L Tricaine
Reagent Final concentration Quantity Tricaine n/a 400 mg 1 M Tris (pH 9) 20 mM 2 mL MilliQ water n/a 98 mL Total n/a 100 mL Note: Adjust to neutral pH by adding acid or base as appropriate until the solution is pH 7. Dilute in embryo medium to 4.2% (v/v).
75% Ethanol
Reagent Final concentration Quantity Ethanol (absolute) 75% 37.5 mL Ultrapure water n/a 12.5 mL Total n/a 50 mL β-glucan peptide stock
Reagent Final concentration Quantity β-glucan peptide 5 mg/mL 5 mg PBS n/a 1 mL Total n/a 1 mL β-glucan control stock
Reagent Final concentration Quantity Control β-glucan (whole glucan peptides, soluble) 5 mg/mL 5 mg PBS n/a 1 mL Total n/a 1 mL β-glucan injection mix (one experiment)
Reagent Final concentration Quantity 5 mg/mL β-glucan peptide (or control) 4 mg/mL 8 μL 0.5% Phenol red 0.1% 2 μL Total n/a 10 μL Note: Make this injection mix fresh for every experimental day. Discard unused mix.
0.5% Phenol red
Reagent Final concentration Quantity Phenol red 0.5% 0.5 g PBS n/a 100 mL Total n/a 100 mL Note: Adjust to neutral pH by adding acid or base as appropriate until the solution is pH 7.
3% methylcellulose
Reagent Final concentration Quantity Methylcellulose 3% 30 g E3 n/a Up to 1 L Total n/a 1 L Note: Shake on an orbital shaker at room temperature for up to one week to dissolve.
iScript cDNA synthesis reaction mix (1 reaction)
Reagent Final concentration Quantity RNA n/a 500 ng (1–15 μL) iScript 5× reaction buffer 1× 4 μL iScript reverse transcriptase n/a 1 μL Ultrapure water n/a To 20 μL Total n/a 20 μL PerfeCTa SYBR Green FastMix with ROX mix (one reaction)
Reagent Final concentration Quantity cDNA (1:5 diluted) n/a 1 μL Forward primer (10 μM) 300 nM 0.3 μL Reverse primer (10 μM) 300 nM 0.3 μL 2× FastMix solution 1× 5 μL Ultrapure water n/a 2.4 μL Total n/a 10 μL
Laboratory supplies
Thin wall borosilicate capillary tubes (Warner Instruments, catalog number: 64-0778)
Small Petri dish (60 mm × 15 mm) (Corning, catalog number: 430166)
Petri dish (150 mm × 25 mm) (Corning, catalog number: 430599)
1.5 mL tube (Axygen, catalog number: MCT-175-C)
0.2 mL PCR tube (Axygen, catalog number: PCR-0208-CP-C)
Dumont #55 forceps (Fine Science Tools, catalog number: 11295-51)
3 mL transfer pipettes (Biologix, catalog number: 30-0138)
384-well plate (Applied Biosystems, catalog number: 4309849)
Microloader pipette tips (0.5–20 μL) (Eppendorf, catalog number: EP5242956003)
1 mL Luer-Lok syringe (BD Biosciences, catalog number: 309628)
27 G needle (0.5 inch) (BD Biosciences, catalog number: 301801)
Equipment
Centrifuge, 2 mL tube rotor (Eppendorf, catalog number: EPP2232000060)
Incubator (set to 28 °C) (Sanyo, catalog number: MIR-162)
Microscope stage micrometre (0.01 mm) (ProSciTech, catalog number: S81K)
Stereomicroscope (Nikon, model: SMZ1500)
Micropipette puller (Sutter Instruments Co., flaming/brown puller set to: heat 680, pull 75, velocity 40, time 55, pressure 530, to produce tapered needles)
Micromanipulator (World Precision Instruments, catalog number: M3301R)
Quantstudio 6 Flex Real-Time PCR System (Thermo Fisher Scientific)
Mastercycler Nexus Thermal Cycler Eco (Eppendorf)
Nanodrop (Thermo Scientific, catalog number: 13-400-518)
Software and datasets
Excel (Microsoft)
Prism v9 (GraphPad)
Procedure
Microinjection of zebrafish larvae
Obtain appropriately staged zebrafish.
Collect eggs by natural spawning from 3–5 pairs of zebrafish. Keep clutches from different breeding pairs separate to enable the collection of data from biological replicates. Collect 30–50 eggs per breeding pair.
Place freshly laid eggs into a clean 150 mm × 25 mm Petri dish with E3 medium.
Observe freshly fertilised eggs under a stereomicroscope. Remove dead eggs (black coagulated material within a chorion) and any other debris (fish waste, fish scales) from the Petri dish.
Place eggs into a 28 °C incubator and leave overnight. Zebrafish were raised in a dark incubator throughout this study.
At 24 hours post fertilisation (hpf), observe embryos under a stereomicroscope and remove dead or underdeveloped embryos. Refer to a zebrafish development chart to assess the developmental stage, such as Kimmel et al. (1995). If transparency is required, supplement embryo medium with 0.003% PTU.
Place embryos in a 28 °C incubator and raise until they are 2.25 dpf/54 hpf.
If unhatched, remove the chorion. Use sharp forceps to puncture and split the chorion open and remove the embryo.
Deliver β-glucan into embryos.
Note: This protocol describes a “double injection” method where embryos/larvae are injected twice with β-glucan, once at 2.25 dpf (54 hpf) and again at 3 dpf (72 hpf), as two injections induce a stronger response than a single injection, as described in Darroch et al. (2022).
Anaesthetise 15–20 embryos at 2.25 dpf (54 hpf) per biological replicate by supplementing embryo medium with 4.2% (v/v) tricaine. Wait for 20–30 s.
Gently touch embryos with a transfer pipette. If they do not twitch or swim away, they are sufficiently anaesthetised.
Pour a small volume (roughly 1 mL) of 3% methylcellulose into a 60 mm × 15 mm Petri dish. Using a microloader pipette tip that has been shortened to 2 cm total length, move the methylcellulose around the Petri dish until it covers the dish in a thin, even layer.
Pool embryos into the middle of the Petri dish by swirling the plate. Pick up embryos using a transfer pipette. Hold the transfer pipette vertical and wait for the embryos to settle at the tip. In one or two drops, place the embryos onto one side of the methylcellulose-coated Petri dish.
Using the shortened microloader tip, array the embryos one by one in a straight line within the Petri dish. Array the embryos laterally with the hindbrain facing right (Figure 1A, 1B, and 1D).
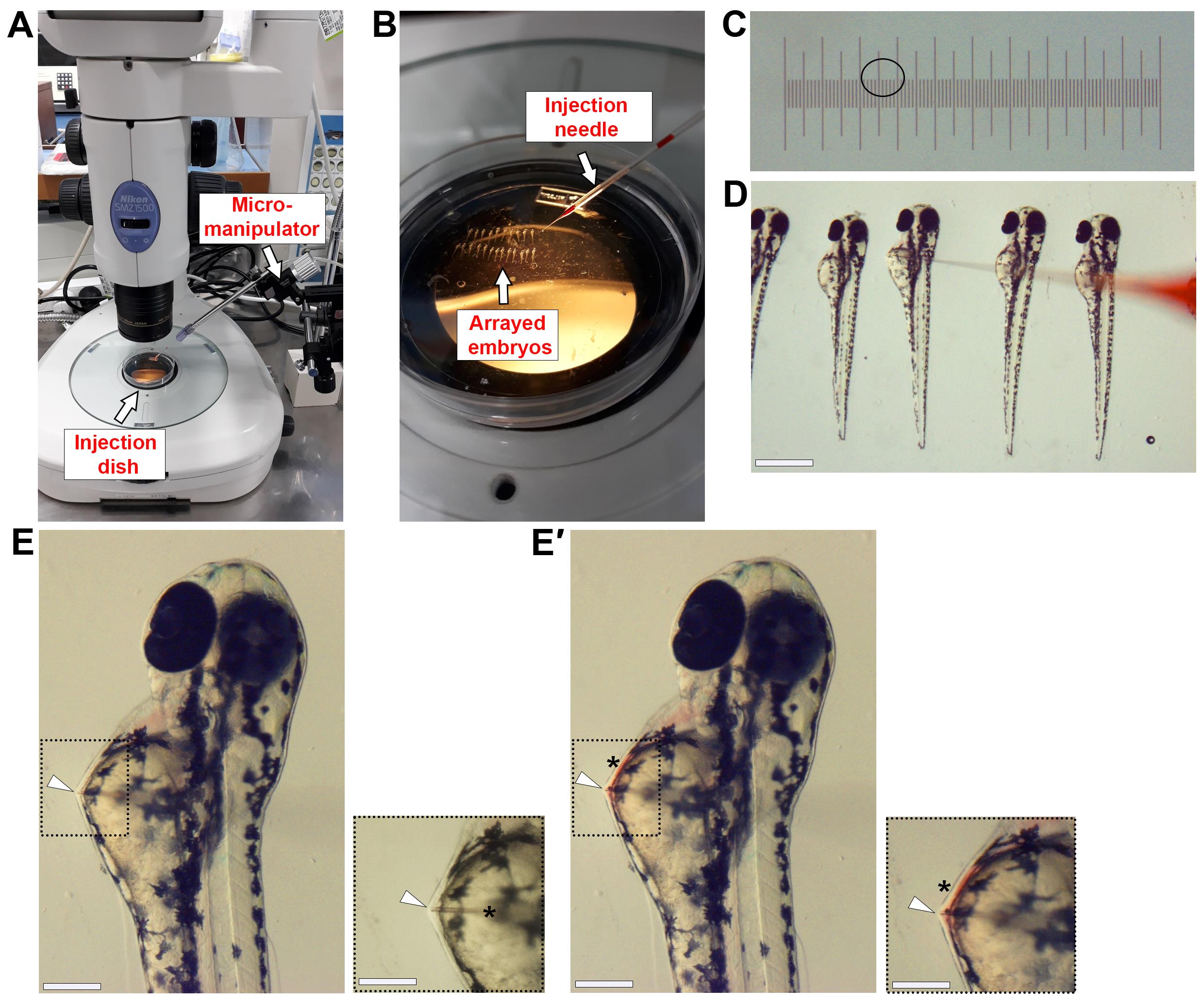
Figure 1. Microinjection of β-glucan into the circulation of zebrafish embryos. (A) Photo showing injection setup. (B) Photo showing embryos arrayed in a Petri dish with microinjection needle in position. (C) Image of 0.01 mm microscope stage slide micrometre. The black circle indicates the size of injection bolus that will deliver 1 nL of solution. (D) Image of 2.25 dpf (54 hpf) embryos arrayed in 3% methylcellulose. The loaded microinjection needle approaches from the right to inject embryos sequentially. (E) Image shows individual embryo with needle positioned for microinjection. The white arrowhead indicates the tip of the needle in the embryo at the correct injection position. Black dashed box is magnified view. In magnified view, the black asterisk indicates the position where the needle enters the yolk sac prior to the injection site. The white arrowhead indicates where the needle can be seen to press against the pericardial wall, which means the needle is deep enough to inject into the circulation. (E′) Image shows the same embryo in (E) immediately following microinjection. The white arrowhead indicates the tip of the needle in the embryo. The black asterisk highlights the red colour of the phenol red dye, indicating successful injection. Black dashed box is magnified view. Scale bars 500 μm in (D), 50 μm in (E, E′), and 40 μm in (E, E′ magnified views).To inject β-glucan, load the microinjection needle (hereafter referred to as needle) with 3 μL of 4 mg/mL β-glucan peptide solution. If injecting the control, load the needle with 3 μL of 4 mg/mL β-glucan control solution.
Under the stereomicroscope, cut the tip of the needle with sharp forceps. The injection mix will move to the tip of the needle after it has been cut.
Calibrate the injection bolus to 1 nL. Add one drop of mineral oil to a 0.01 mm microscope stage slide micrometre and, under a stereomicroscope, focus on the ruler. Adjust the pressure and pulse duration of the injection box until the injection bolus measures 1.2 units (Figure 1C). This is 1 nL.
Inject 1 nL (4 ng) of β-glucan into the circulation of embryos (Figure 1D and 1E). Approaching from the right, pierce through the yolk sac of each embryo and move the needle towards the pericardial cavity until you see the needle touch the pericardial wall. Once in position, inject. The red dye in the injection mix indicates successful injection, as the dye gets taken up into circulation as the heart beats (Figure 1E′).
Note: Do not pierce through the epidermal wall.
See Troubleshooting.
Pour enough E3 medium into the injection Petri dish to completely cover the embryos. Using a transfer pipette, suck up and eject E3 repeatedly over the embryos until they dislodge from the methylcellulose. Gently pick up the injected embryos with a transfer pipette and place into a new 150 mm × 25 mm Petri dish with fresh E3 medium.
Place the Petri dish into a 28 °C incubator and leave overnight.
At 3 dpf (72 hpf), repeat steps 2a–2k to deliver a second dose of β-glucan into the larvae. This second dose is 8 ng, so inject 2 nL into the larvae by injecting 1 nL twice in rapid succession.
Analysis of gene expression post injection
Extract total RNA from injected larvae.
At the desired time post injection, anaesthetise pools of 15–20 larvae per biological replicate by supplementing the embryo medium with 4.2% (v/v) tricaine. We investigated gene expression at 1, 4, and 11 days post injection (dpi) with three biological replicates per time point (Figure 2A).
Transfer pooled larvae into 1.5 mL microcentrifuge tubes.
Remove as much E3 as possible using a transfer pipette. A small volume (< 100 μL) of residual E3 will not disrupt downstream processes.
Pipette 1 mL of TRIzol reagent into the 1.5 mL tubes.
Caution: TRIzol is hazardous and should be handled in a fume hood.
Connect a 27-gauge needle to a sterile 1 mL syringe. Carefully aspirate the larvae into the syringe and dispense back into the 1.5 mL tube. Repeat until the larval tissue is homogenised.
Incubate for 5 min at room temperature.
Pause point: Store samples at -80 °C or continue immediately.
Add 200 μL of chloroform and vortex twice for 5 s.
Incubate for 3 min at room temperature.
Centrifuge at 12,000× g for 15 min at 4 °C.
Observe how the sample has split into three layers with a clear upper layer (phase), middle white interphase, and lower pink phase. Without touching the white or pink phases, carefully remove the upper aqueous phase and transfer into a new 1.5 mL microcentrifuge tube.
Add 500 μL of 100% isopropanol and vortex for 1 min.
Incubate for 10 min at room temperature.
Centrifuge at 12,000× g for 10 min at 4 °C.
Remove the supernatant by pouring and observe the white pellet.
Add 1 mL of 75% ethanol onto the pellet.
Spin at 7,500× g for 5 min at 4 °C.
Remove all ethanol by pipetting and allow the pellet to air dry for 5 min at room temperature.
Resuspend the pellet in 30 μL of ultrapure water. Keep on ice.
Quantify RNA concentration by nanodrop.
Pause point: Store RNA at -80 °C or continue immediately.
Real-time quantitative PCR (RT qPCR) expression analysis of genes of interest.
Reverse transcribe cDNA using the iScript cDNA Synthesis kit. In a 0.2 mL tube, prepare the iScript cDNA synthesis reaction mix (Recipe 9).
Place samples into an Eppendorf Mastercycler Nexus Thermal Cycler Eco and run the following protocol: 25 °C for 5 min, 46 °C for 20 min, 95 °C for 1 min.
Remove from the thermal cycler and place on ice.
Pause point: Store cDNA at -80 °C or continue immediately.
Add 80 μL of ultrapure water to the 20 μL cDNA reaction to dilute the cDNA 1:5.
For the qPCR reaction, prepare the PerfeCTa SYBR Green FastMix with ROX mix (Recipe 10).
Pipette into a 384-well plate with three technical replicates for each sample per gene.
Place the reaction into a Quantstudio 6 Flex Real-Time PCR System and run the following cycling parameters: initial denaturation 95 °C for 2 min, followed by 40 cycles at 95 °C for 15 s, 60 °C for 30 s.
Record the Ct values of each gene and proceed to data analysis (Figure 2B).
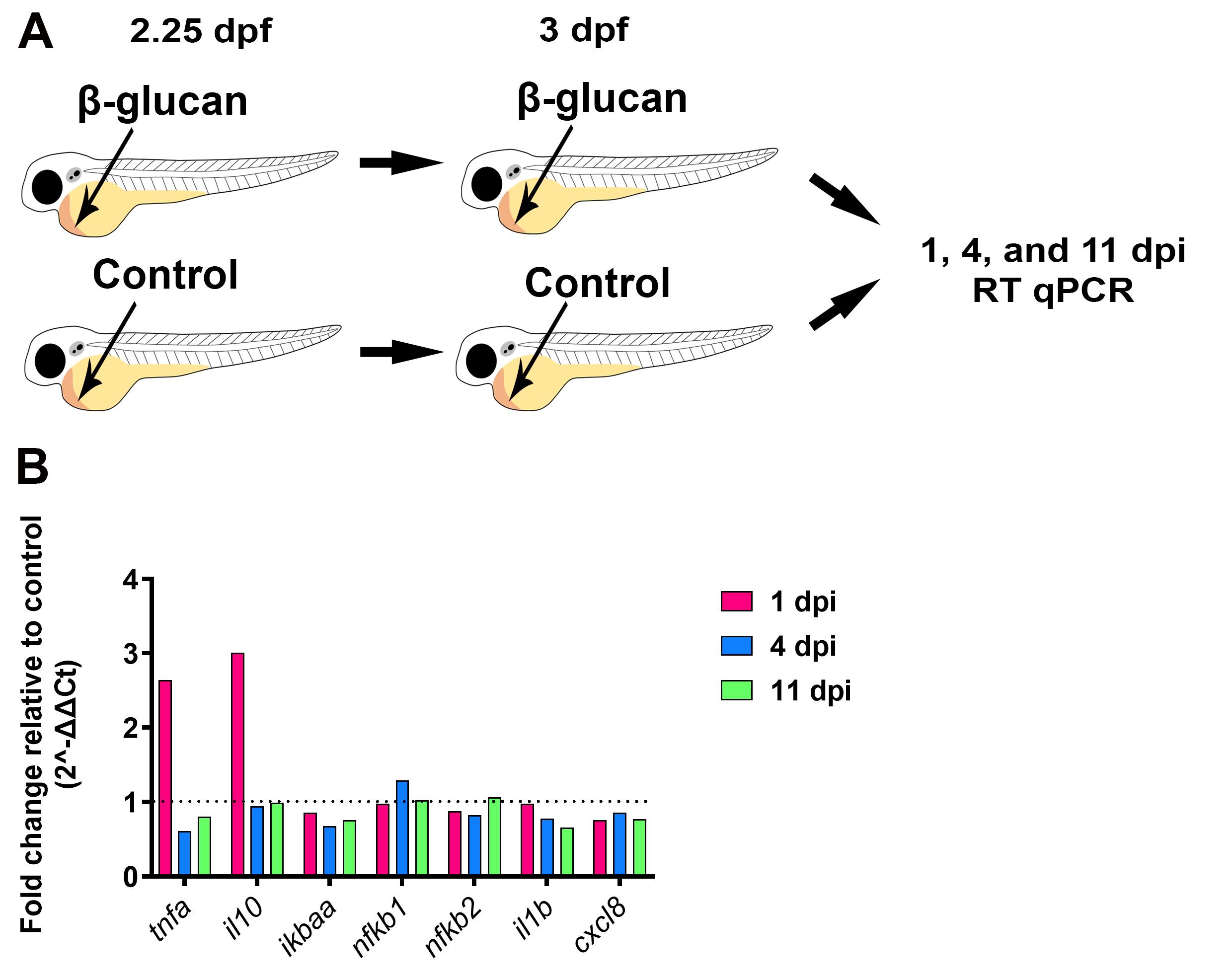
Figure 2. Gene expression analysis following β-glucan injection. (A) Schematic illustrating the injection protocol followed by time points of sample collection for subsequent RT qPCR. (B) Fold change of relevant immune genes at 1, 4, and 11 days post injection (dpi) with β-glucan relative to control. Data shown is from averaged ΔCt values from three biological replicates, n = 15 larvae per biological replicate. Endogenous control gene was ef1a.
Data analysis
Microsoft Excel was used to analyse the data with ef1a as the endogenous control (reference) gene. Briefly, the ΔCt value was calculated by subtracting the average Ct value of the reference gene from the average Ct value of the gene of interest (Ctaverage gene of interest – Ctaverage ef1a) (Livak and Schmittgen, 2001). The ΔΔCt value was calculated by subtracting the ΔCt value of the average ΔCt of the control samples from the average ΔCt of the experimental samples (i.e., β-glucan injected) (ΔCtaverage β-glucan injected – ΔCtaverage control). Gene-expression fold change was determined by the 2-ΔΔCT method (Livak and Schmittgen, 2001). Data was visualised using GraphPad Prism version 8.
Validation of protocol
Previous studies showed that the NF-kB pathway is involved in the response to β-glucan, and the expression of specific inflammatory cytokines such as il1b, tnfa, cxcl8, and il10 are upregulated following stimulation (Rogers et al., 2005; Inoue and Shinohara, 2014; Megías et al., 2016). The RT qPCR data presented in this current study shows that two predicted genes of interest were upregulated following β-glucan injection when compared to control, and this analysis was further characterised in the recent publication (Darroch et al., 2022). Darroch et al. (2022) utilised this method to stimulate a trained-like phenotype in larval zebrafish that protected larvae from subsequent bacterial challenge and increased neutrophil recruitment to infection.
General notes and troubleshooting
General notes
Larval zebrafish offer a unique opportunity to investigate trained innate responses entirely in vivo. On a whole animal scale, post-training phenotypes such as larval survival and pathogen clearance can be assessed following live infection as demonstrated in Darroch et al. (2022). Such analyses are enhanced with the use of fluorescent pathogens that can be visualised within transparent larvae using fluorescent microscopy. A wide range of pathogens or sterile insults can be used to challenge larvae post training to assess changes in innate immune function (Linnerz and Hall, 2020). In addition, there are numerous transgenic reporter lines available with fluorescently marked innate immune cells that can be directly observed in real time, at single-cell resolution (Astin et al., 2017; Linnerz and Hall, 2020). Analysis of phagocyte functions such as bacterial killing capacity, recruitment to infection/inflammation, or production of anti-microbial molecules (e.g., reactive intermediate species, as detected by fluorescent probes) can be assessed post training (Astin et al., 2017, Darroch et al., 2022).
Troubleshooting
Needle blocking: as the β-glucan is insoluble, it can block the needle. If the needle is blocked, increase the air pressure of the injector, change the mode of the injector to continuous airflow, and attempt to flush the β-glucan clumps out of the needle. If this does not work, you can cut the needle slightly larger and try to flush it again. You should not make the needle larger indefinitely; when the bore of the needle gets to a size that damages the larvae, you will need to load and cut a new needle.
Acknowledgments
This work was supported by grants awarded to CJH from Marsden Fund, Royal Society of New Zealand (grant # 3706135) and Health Research Council of New Zealand (grant # 17/294). This protocol was initially described and validated in Darroch et al. (2022).
Competing interests
The authors declare that they have no competing interests.
Ethical considerations
All zebrafish research was conducted with the approval of the University of Auckland Animal Ethics Committee (approval numbers AEC001911 and AEC22563).
References
- Arts, R. J., Carvalho, A., La Rocca, C., Palma, C., Rodrigues, F., Silvestre, R., Kleinnijenhuis, J., Lachmandas, E., Gonçalves, L. G., Belinha, A., et al. (2016). Immunometabolic Pathways in BCG-Induced Trained Immunity. Cell Rep. 17(10): 2562–2571.
- Arts, R. J., Moorlag, S. J., Novakovic, B., Li, Y., Wang, S. Y., Oosting, M., Kumar, V., Xavier, R. J., Wijmenga, C., Joosten, L. A., et al. (2018). BCG Vaccination Protects against Experimental Viral Infection in Humans through the Induction of Cytokines Associated with Trained Immunity. Cell Host Microbe 23(1): 89–100.e5.
- Astin, J. W., Keerthisinghe, P., Du, L., Sanderson, L. E., Crosier, K. E., Crosier, P. S. (2017). Chapter 2 - Innate immune cells and bacterial infection in zebrafish. Method Cell Bio 13: 31–60.
- Darroch, H., Astin, J. W. and Hall, C. J. (2022). Towards a new model of trained immunity: Exposure to bacteria and β-glucan protects larval zebrafish against subsequent infections. Dev Comp Immunol 132: 104400.
- Ferreira, A. V., Kostidis, S., Groh, L. A., Koeken, V. A., Bruno, M., Baydemir, I., Kilic, G., Bulut, Ã., Andriopoulou, T., Spanou, V., et al. (2023). Dimethyl itaconate induces long-term innate immune responses and confers protection against infection. Cell Rep. 42(6): 112658.
- Hamada, A., Torre, C., Drancourt, M. and Ghigo, E. (2019). Trained Immunity Carried by Non-immune Cells. Front. Microbiol. 9: e03225.
- Inoue, M. and Shinohara, M. L. (2014). Clustering of Pattern Recognition Receptors for Fungal Detection. PLoS Pathog. 10(2): e1003873.
- Kalafati, L., Kourtzelis, I., Schulte-Schrepping, J., Li, X., Hatzioannou, A., Grinenko, T., Hagag, E., Sinha, A., Has, C., Dietz, S., et al. (2020). Innate Immune Training of Granulopoiesis Promotes Anti-tumor Activity. Cell 183(3): 771–785.e12.
- Kaufmann, E., Sanz, J., Dunn, J. L., Khan, N., Mendonça, L. E., Pacis, A., Tzelepis, F., Pernet, E., Dumaine, A., Grenier, J. C., et al. (2018). BCG Educates Hematopoietic Stem Cells to Generate Protective Innate Immunity against Tuberculosis. Cell 172: 176–190.e19.
- Kimmel, C. B., Ballard, W. W., Kimmel, S. R., Ullmann, B. and Schilling, T. F. (1995). Stages of embryonic development of the zebrafish. Dev. Dyn. 203(3): 253–310.
- Kleinnijenhuis, J., Quintin, J., Preijers, F., Joosten, L. A. B., Ifrim, D. C., Saeed, S., Jacobs, C., van Loenhout, J., de Jong, D., Stunnenberg, H. G., et al. (2012). Bacille Calmette-Guérin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc. Natl. Acad. Sci. U.S.A. 109(43): 17537–17542.
- Lam, S., Chua, H., Gong, Z., Lam, T. and Sin, Y. (2004). Development and maturation of the immune system in zebrafish, Danio rerio: a gene expression profiling, in situ hybridization and immunological study. Dev. Comp. Immunol 28(1): 9–28.
- Linnerz, T. and Hall, C. J. (2020). The Diverse Roles of Phagocytes During Bacterial and Fungal Infections and Sterile Inflammation: Lessons From Zebrafish. Front. Immunol. 11: e01094.
- Livak, K. J. and Schmittgen, T. D. (2001). Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 25(4): 402–408.
- Medina-Gali, R. M., Ortega-Villaizan, M. d. M., Mercado, L., Novoa, B., Coll, J. and Perez, L. (2018). Beta-glucan enhances the response to SVCV infection in zebrafish. Dev. Comp. Immunol 84: 307–314.
- Megías, J., Martínez, A., Yáñez, A., Goodridge, H. S., Gozalbo, D. and Gil, M. L. (2016). TLR2, TLR4 and Dectin-1 signalling in hematopoietic stem and progenitor cells determines the antifungal phenotype of the macrophages they produce. Microbes and Infection 18(5): 354–363.
- Mitroulis, I., Ruppova, K., Wang, B., Chen, L. S., Grzybek, M., Grinenko, T., Eugster, A., Troullinaki, M., Palladini, A., Kourtzelis, I., et al. (2018). Modulation of Myelopoiesis Progenitors Is an Integral Component of Trained Immunity. Cell 172: 147–161.e12.
- Netea, M. G., Domínguez-Andrés, J., Barreiro, L. B., Chavakis, T., Divangahi, M., Fuchs, E., Joosten, L. A. B., van der Meer, J. W. M., Mhlanga, M. M., Mulder, W. J. M., et al. (2020). Defining trained immunity and its role in health and disease. Nat. Rev. Immunol. 20(6): 375–388.
- Rodríguez, I., Chamorro, R., Novoa, B. and Figueras, A. (2009). β-Glucan administration enhances disease resistance and some innate immune responses in zebrafish (Danio rerio). Fish Shellfish Immunol 27(2): 369–373.
- Roesner, L. M., Ernst, M., Chen, W., Begemann, G., Kienlin, P., Raulf, M. K., Lepenies, B. and Werfel, T. (2019). Human thioredoxin, a damage-associated molecular pattern and Malassezia-crossreactive autoallergen, modulates immune responses via the C-type lectin receptors Dectin-1 and Dectin-2. Sci. Rep. 9(1): 11210.
- Rogers, N. C., Slack, E. C., Edwards, A. D., Nolte, M. A., Schulz, O., Schweighoffer, E., Williams, D. L., Gordon, S., Tybulewicz, V. L., Brown, G. D., et al. (2005). Syk-Dependent Cytokine Induction by Dectin-1 Reveals a Novel Pattern Recognition Pathway for C Type Lectins. Immunity 22(4): 507–517.
- Silliman, C. C. and Wang, M. (2006). The merits of in vitro versus in vivo modeling in investigation of the immune system. Environ. Toxicol. Pharmacol. 21(2): 123–134.
Article Information
Publication history
Published: Dec 5, 2023
Copyright
© 2023 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
How to cite
Darroch, H., Astin, J. W. and Hall, C. J. (2023). Microinjection of β-glucan Into Larval Zebrafish (Danio rerio) for the Assessment of a Trained-Like Immunity Phenotype. Bio-protocol 13(23): e4888. DOI: 10.21769/BioProtoc.4888.
Category
Immunology > Animal model > Zebrafish
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Tips for asking effective questions
+ Description
Write a detailed description. Include all information that will help others answer your question including experimental processes, conditions, and relevant images.
Share
Bluesky
X
Copy link