- Submit a Protocol
- Receive Our Alerts
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Purification of Human Cytoplasmic Actins From Saccharomyces cerevisiae
Published: Vol 13, Iss 23, Dec 5, 2023 DOI: 10.21769/BioProtoc.4894 Views: 94
Reviewed by: William Jennings ValentineAlexandros C KokotosAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols
Efficient Generation of Genome-wide Libraries for Protein–ligand Screens Using Gibson Assembly
Tamara Sternlieb [...] Igor Cestari
Nov 20, 2022 1267 Views
Lysate-to-grid: Rapid Isolation of Native Complexes from Budding Yeast for Cryo-EM Imaging
Ian Cooney [...] Peter S. Shen
Jan 20, 2023 992 Views
Optimized Expression and Isolation of Recombinant Active Secreted Proteases Using Pichia pastoris
Adam Turner [...] Angie Gelli
Mar 5, 2023 791 Views
Abstract
Eukaryotic cells rely on actin to support cellular structure, motility, transport, and a wide variety of other cytoplasmic functions and nuclear activities. Humans and other mammals express six closely related isoforms of actin, four of which are found primarily in skeletal, cardiac, and smooth muscle tissues. The final two isoforms, β and γ, are found in non-muscle cells. Due to the ease of purification, many biochemical studies surveying the functions of actin and its regulators have been carried out with protein purified from skeletal muscle. However, it has become increasingly clear that some activities are isoform specific, necessitating more accessible sources of non-muscle actin isoforms. Recent innovations permit the purification of non-muscle actins from human cell culture and heterologous systems, such as insect cell culture and the yeast Pichia pastoris. However, these systems generate mixtures of actin types or require additional steps to remove purification-related tags. We have developed strains of Saccharomyces cerevisiae (budding yeast) that express single untagged isoforms of either human non-muscle actin (β or γ) as their sole actin, allowing the purification of individual homogeneous actin isoforms by conventional purification techniques.
Key features
• Easy growth of yeast as a source of human cytoplasmic actin isoforms.
• Uses well-established actin purification methods.
• The tag-free system requires no post-purification processing.
Graphical overview
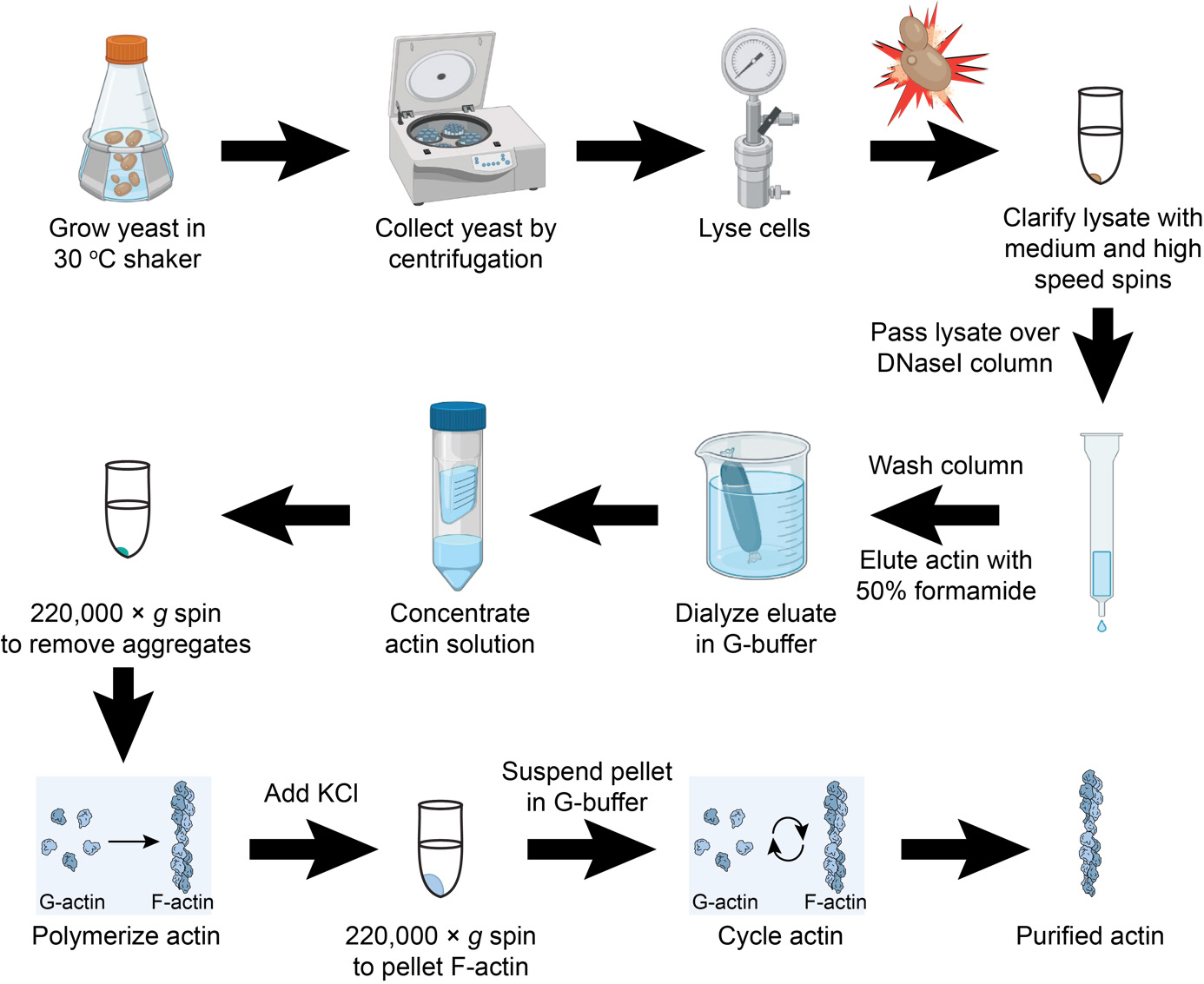
Isolating human cytoplasmic actins from yeast
Keywords: Actin purificationBackground
Actin and its many regulators are critical to a wide variety of eukaryotic cellular functions, ranging from determination of cell shape and motility to critical nuclear functions that include gene regulation and DNA repair (Belin et al., 2015; Schaks et al., 2019; Kadzik et al., 2020). In humans, the importance of actin regulation is further implicated in cancer metastasis, cardiomyopathies, neurological dysfunctions, and many other debilitating disorders (Rivière et al., 2012; Rubenstein and Wen, 2014; Bamburg and Bernstein, 2016; Hyrskyluoto and Vartiainen, 2020; Izdebska et al., 2020). However, mechanistic advances linking actin to these maladies have been confounded by the presence of six closely related yet distinct actin isoforms. Four actin isoforms are found predominantly in skeletal, cardiac, and smooth muscle tissues (α1, α2, α-cardiac, and γ2), and two are found primarily in non-muscle cells (β and γ1, commonly referred to as cytoplasmic actins). Further, much of what we know about the biochemical nature of critical actin regulators has been deciphered using the readily abundant and easily obtained 1 actin from animal muscle sources (e.g., rabbit, chicken, or cow). While muscle actin has been invaluable for the study of actin organization, regulation, and dynamics, it is becoming increasingly clear that some actin regulators display isoform specificity (De La Cruz, 2005; Perrin and Ervasti, 2010; Antoku et al., 2019). Thus, reliable sources of pure cytoplasmic actin isoforms are required to effectively explore these differing functions. However, unlike the muscle actin isoform α1, these isoforms are difficult to obtain in suitable concentrations and purity for widespread adoption and use.
Initially developed methods obtained mixtures of cytoplasmic actins from human cells (Schafer et al., 1998); similar mixtures of β and γ1 are available from commercial sources (Cytoskeleton, Inc., Denver, CO). Newer systems involving cleavable fusion proteins expressed in human cells or in the yeast Pichia pastoris improved purity to individual isoforms and extended expression to disease-relevant mutations in actin isoforms (Hatano et al., 2018; Hatano et al., 2020; Ceron et al., 2022). These purification systems have the further benefit of extending isoform analysis to important actin post-translational modifications, such as His73 methylation, and N-terminal processing. We produced a different, relatively inexpensive system that yields Human β or γ1 actin from the budding yeast Saccharomyces cerevisiae. Here, we combine engineered yeast strains with the classic purification of actin via DNase I affinity and elution with the denaturant formamide (Zechel et al., 1980; Kilmartin and Adams 1984; Kron et al., 1992; Goode 2002). This provides a relatively simple technique that does not require additional column chromatography. These and related sources of human cytoplasmic actins (Hatano et al., 2018; Hatano et al., 2020; Ceron et al., 2022) will play an important role in understanding the biochemical activities of non-muscle actin regulatory proteins, which may provide important new insights into actin-based disease development and progression.
Materials and reagents
Biological materials
BHY845 [MATa ura3∆0 leu2∆0 his3∆1 act1∆::NatR (pBH839 = 2μ LEU2 GPDpr-HsACTB)] S. cerevisiae strain that expresses only human β actin (Haarer et al., 2023); available with material transfer agreement (MTA) due to pending patent application (see conflict of interest statement).
BHY848 [MATα ura3∆0 leu2∆0 his3∆1 mfa1∆:: MFA1pr-SpHIS5 act1∆::NatR (pBH869 = 2μ LEU2 GPDpr-HsACTG1)] S. cerevisiae strain that expresses only human γ actin (Haarer et al., 2023); available on request, as above.
Reagents
Bacto peptone (BD-Gibco, catalog number: 211677)
Bacto yeast extract (BD-Gibco, catalog number: 212750)
Bacto agar (BD-Difco, catalog number: 214010)
Glucose (Sigma, catalog number: G-7021; Mallinckrodt, catalog number: 4908)
Glacial acetic acid (Fisher, catalog number: A38-212)
Hydrochloric acid (HCl) (Fisher, catalog number: A144S-212)
Tris base (Fisher, BP152-5) or Trizma base (Sigma, catalog number: T6066)
Sodium acetate (J.T. Baker, catalog number: 3470-01; Fisher, catalog number: BP333-500)
Sodium chloride (NaCl) (Sigma, catalog number: S7653)
Sodium bicarbonate (NaHCO3) (Sigma, catalog number: S5761)
Formamide, deionized (Sigma, catalog number: F9037)
Ammonium chloride (NH4Cl) (Sigma, catalog number: A9434)
Potassium chloride (KCl) (J.T. Baker, catalog number: 3040-01)
Calcium chloride dihydrate (CaCl2·2H2O) (Fisher, catalog number: BP510-500)
CNBr-activated Sepharose 4B (Cytiva, catalog number: 17-0430-01)
Deoxyribonuclease I (DNase I) (Worthington, catalog number: LS002007)
Bio-Rad protein assay dye reagent (Bio-Rad, catalog number: 5000006)
Calbiochem protease inhibitor cocktail IV (Millipore-Sigma, catalog number: 539136)
Phenylmethylsulfonyl fluoride (PMSF) (Sigma, catalog number: P7626)
Magnesium chloride, 6-hydrate (MgCl2·6H2O) (J.T. Baker, catalog number: 4003-01)
Ethylene glycol-bis(2-aminoethylether)-N,N,N′,N′-tetraacetic acid (EGTA) (Sigma, catalog number: E4378)
Adenosine 5′-triphosphate, disodium trihydrate (ATP) (GoldBio, catalog number: A-081-25)
Dithiothreitol (DTT) (Bio-Rad, catalog number: 161-0611; GoldBio, catalog number: DTT25)
Potassium hydroxide (KOH) (Fisher, catalog number: P250-500)
Sodium hydroxide (NaOH) (Fisher, catalog number: BP359-500)
Sodium azide (Fisher, catalog number: BP922I-500)
Solutions
40% (w/v) glucose
1 mM HCl
1 M Tris pH 8.0
1 M Tris pH 7.5
3 M KCl
1 M MgCl2
0.5 M CaCl2
5 M NaCl
2% sodium azide
YPD medium (1 L), liquid (see Recipes)
YPD medium (1 L), plates (see Recipes)
40% glucose (1 L) (see Recipes)
Coupling buffer (250 mL) (see Recipes)
Low pH wash buffer (100 mL) (see Recipes)
High pH wash buffer (100 mL) (see Recipes)
0.5 M sodium acetate, pH 4 (500 mL) (see Recipes)
1 M tris, pH 7.5 or pH 8.0 (1 L) (see Recipes)
DNase I storage buffer (100 mL) (see Recipes)
G-buffer (50 mL) (see Recipes)
Lysis buffer (50 mL) (see Recipes)
Actin wash buffer 1 (50 mL) (see Recipes)
Actin wash buffer 2 (50 mL) (see Recipes)
Actin elution buffer (20 mL) (see Recipes)
20× F-buffer (1 mL) (see Recipes)
100 mM PMSF (100 mL) (see Recipes)
0.5 M ATP (20 mL) (see Recipes)
1 M DTT (50 mL) (see Recipes)
0.5 M EGTA (100 mL) (see Recipes)
Recipes
YPD medium (1 L), liquid
Reagent Final concentration Quantity Bacto peptone 2% (w/v) 20 g Bacto yeast extract 1% (w/v) 10 g H2O 900 mL Glucose (dextrose) 4% (w/v) 100 mL of 40% stock (see Note*, below) *Note: Autoclave the peptone and yeast extract solution separately from the 40% glucose solution. This limits media caramelization and reaction with media components. Thus, to complete the YPD, add the sterile peptone-yeast solution to the sterile glucose aseptically (i.e., 900 mL of peptone-yeast solution and 100 mL of 40% glucose). Separate media components can be stored for several weeks at room temperature or at 4 °C for longer periods.
YPD medium (1 L), plates
Reagent Final concentration Quantity Bacto peptone 2% (w/v) 20 g Bacto yeast extract 1% (w/v) 10 g Bacto agar 1.8% (w/v) 18 g (see note*) H2O 900 mL Glucose (dextrose) 4% (w/v) 100 mL of 40% stock (see Note*, below) *Note: For plates, add agar to peptone and yeast extract solution, add magnetic stir bar, and autoclave. After autoclaving, add sterile 40% glucose solution and place on stir plate with slow stirring to avoid bubbles; let cool to ~55 °C. Carefully pour media plus agar solution into (35–40) sterile Petri dishes (10 cm) and let sit on bench for 1–2 days at room temperature; plates can be stored upside down in sealed Petri dish bags for several weeks at room temperature or at 4 °C for longer periods.
40% glucose (1 L)
Filter sterilize or autoclave
Reagent Quantity Glucose (dextrose) 400 g H2O to 1 L Coupling buffer (250 mL)
Reagent Final concentration Stock solution Quantity Sodium bicarbonate 0.1 M 2.1 g Sodium chloride 0.5 M 5 M 25 mL Calcium chloride 0.1 mM 0.5 M 50 μL H2O Final volume to 250 mL Low pH wash buffer (100 mL)
Reagent Final concentration Stock solution Quantity Sodium acetate, pH 4 0.1 M 0.5 M, pH 4 20 mL Sodium chloride 0.5 M 5 M 10 mL H2O 70 mL High pH wash buffer (100 mL)
Reagent Final concentration Stock solution Quantity Tris, pH 8 0.1 M 1 M, pH 8 10 mL Sodium chloride 0.5 M 5 M 10 mL H2O 80 mL 0.5 M sodium acetate, pH 4 (500 mL)
Reagent Quantity Sodium acetate 20.5 g Glacial acetic acid to pH 4.0 H2O to 500 mL 1 M tris, pH 7.5 or pH 8.0 (1 L)
Reagent Quantity Tris base 121.1 g HCl to pH 7.5 or 8.0 H2O to 1 L DNase I storage buffer (100 mL)
Reagent Final concentration Stock solution Quantity Tris, pH 7.5 50 mM 1 M 5 mL Sodium chloride 0.5 M 5 M 10 mL Sodium azide 0.02% (w/v) 2% (w/v) 1 mL H2O 84 mL G-buffer (50 mL)
Reagent Final concentration Stock solution Quantity Tris, pH 7.5 10 mM 1 M 0.5 mL Calcium chloride 0.2 mM 0.5 M 20 μL ATP 0.5 mM 0.5 M 50 μL (see Note*, below) DTT 0.2 mM 1 M 10 μL* H2O 49.4 mL* *Note: Add ATP and DTT solutions the day of use; store G-buffer with ATP and DTT on ice or at 4 °C.
Lysis buffer (G-buffer and protease inhibitors; 50 mL)
Reagent Final concentration Stock solution Quantity Tris, pH 7.5 10 mM 1 M 0.5 mL Calcium chloride 0.2 mM 0.5 M 20 μL ATP 0.5 mM 0.5 M 50 μL DTT 0.2 mM 1 M 10 μL PMSF 0.1 mM 0.1 M 50 μL Calbiochem protease inhibitor cocktail IV 0.2% (v/v) 100 μL H2O 49.27 mL Actin wash buffer 1 (G-buffer and 10% formamide; 50 mL)
Reagent Final concentration Stock solution Quantity Tris, pH 7.5 10 mM 1 M 0.5 mL Calcium chloride 0.2 mM 0.5 M 20 μL ATP 0.5 mM 0.5 M 50 μL DTT 0.2 mM 1 M 10 μL PMSF 0.1 mM 0.1 M 50 μL Formamide 10% (v/v) 5 mL H2O 44.37 mL Actin wash buffer 2 (G-buffer and 0.2 M NH4Cl; 50 mL)
Reagent Final concentration Stock solution Quantity Tris, pH 7.5 10 mM 1 M 0.5 mL Calcium chloride 0.2 mM 0.5 M 20 μL ATP 0.5 mM 0.5 M 50 μL DTT 0.2 mM 1 M 10 μL Ammonium chloride 0.2 M 0.5 M 20 mL H2O 29.42 mL Actin elution buffer (G-buffer and 50% formamide; 20 mL)
Reagent Final concentration Stock solution Quantity Tris, pH 7.5 10 mM 1 M 0.2 mL Calcium chloride 0.2 mM 0.5 M 8 μL ATP 0.5 mM 0.5 M 20 μL DTT 0.2 mM 1 M 4 μL Formamide 50% (v/v) 10 mL H2O 9.77 mL 20× F-buffer (1 mL)
10 mM tris pH 7.5, 500 mM KCl, 80 mM MgCl2, 20 mM EGTA, 10 mM ATP
Reagent Final concentration Stock solution Quantity Tris, pH 7.5 10 mM 1 M 10 μL Potassium chloride 0.5 M 3 M 167 μL Magnesium chloride 80 mM 1 M 80 μL EGTA 20 mM 0.5 M 40 μL ATP 10 mM 0.5 M 20 μL H2O 683 μL 100 mM PMSF (100 mL)
Store at -20 °C
Reagent Quantity PMSF 1.74 g 100% ethanol to 100 mL 0.5 M ATP (20 mL)
Store filter-sterilized frozen aliquots at -20 °C
Reagent Quantity ATP 6.05 g KOH to pH ~ 7.2 H2O to 20 mL 1 M DTT (50 mL)
Store filter-sterilized frozen aliquots at -20 °C
Reagent Quantity DTT 7.71 g H2O to 50 mL 0.5 M EGTA (100 mL)
Reagent Quantity EGTA
NaOH
19.0 g
to pH 8
H2O to 100 mL
Laboratory supplies
50 mL conical tubes (Thermo Fisher Scientific, Falcon, catalog number: 1495949A)
15 mL conical tubes (Thermo Fisher Scientific, Falcon, catalog number: 1495949B)
Pierce 5 mL disposable columns, including polyethylene discs, stoppers, and end caps (Thermo/Pierce, catalog number: 29922)
Funnels for Pierce columns (Thermo/Pierce, catalog number: 29923)
Petri dishes (Kord-Valmark, catalog number: 2900)
2 L Erlenmeyer flasks
Syringe filters, 0.45 μm (Genesee Scientific, catalog number: 25-242)
Slide-A-Lyzer dialysis cassettes, 7,000 or 10,000 MWCO (Thermo Fisher Scientific, catalog numbers: 66370 and 66380, respectively)
Dialysis tubing, 12,000–14,000 MWCO, 25 mm width (Fisher Scientific, catalog number: 21-152-18)
Protein concentrators: Vivaspin-6, 5,000 MWCO (Sartorius, catalog number: VS0611); Amicon Ultra-4, 10,000 MWCO (Millipore-Sigma, catalog number: UFC801024)
Equipment
Magnetic stir plate (optional; for making YPD plates)
Shaking incubator capable of holding multiple 2 L flasks at 30 °C
Spectrophotometer for cell density measurement (Bio-Rad, model: SmartSpec 3000)
Rocker or rotator, preferably at 4 °C (e.g., in cold room or chromatography cabinet)
Centrifuges:
Refrigerated tabletop centrifuge (Beckman Coulter, model: Allegra X-15R) for preparing resin
Superspeed centrifuge (Thermo Scientific/Sorvall, model: Lynx 4000) and rotor (Thermo Scientific, model: F10-4x1000 LEX) for harvesting 1–2 L of yeast
Ultracentrifuge (Beckman Coulter, model: Optima LE-80K) and rotor (Beckman Coulter, model: 70 Ti) for clarifying yeast lysates
Tabletop ultracentrifuge (Beckman Coulter, model: Optima Max-XP) and rotors (Beckman Coulter, models: TLA100, TLA100.2, TLA100.3) for clarifying actin solutions
French press and pressure cell with 1-inch diameter piston (American Instrument Company/SIM-Aminco)
Protein gel apparatus and power supply (Bio-Rad, models: Mini-Protean Tetra, PowerPac Basic Power Supply)
Optional: spectrophotometer for determining protein concentration (Thermo Scientific, model: NanoDrop 2000)
Procedure
Cell growth
Streak strains BHY845 and BHY848 (expressing β- and γ1-actin, respectively) from frozen glycerol stocks onto YPD plates (4% glucose) and incubate at 30 °C until colonies form (4–6 d; these strains are particularly slow growing, with doubling times ~4–6 times longer than wild-type laboratory strains).
Inoculate flasks containing 50 mL of YPD (4% glucose) with several colonies for each strain; grow on a shaker overnight at 30 °C.
Use 25–40 mL of the cultures from step A2 to inoculate 1 L of YPD cultures; incubate on a shaker at 30 °C.
Grow strains to OD595 ~1.5–2, which may take ~1.5–2 days.
Harvest yeast cells by centrifugation, e.g., in 250 mL bottles using a Beckman JA12 rotor at 7,000 rpm (7,900× g) for 7 min.
Decant culture medium and resuspend cells (e.g., by vortexing) in 10 mM Tris, pH 7.5, 0.2 mM CaCl2; transfer to 50 mL conical tubes. Pellets typically fill 6–7 mL of space in the tube.
Spin at 3,500× g for 10 min at 4 °C, decant, and then freeze pellets at -80 °C.
Coupling DNase I to Sepharose 4B (DNase I-resin preparation)
Swell 3 g of CNBr-activated Sepharose 4B in ice-cold 1 mM HCl in a 50 mL conical tube; place on a rocker or rotator to disperse persistent clumps.
Collect activated beads via centrifugation at ~2,000× g for 2 min; wash twice with 1 mM HCl and bring volume to 50 mL with each wash.
Resuspend 100 mg of DNase I in coupling buffer at 5 mg/mL final concentration; save a small aliquot (~100 μL) of DNase I suspension for later calculation of coupling efficiency and add the remainder to the resin. Incubate coupling reaction overnight at 4 °C on a rocker or rotator.
Allow the resin to settle on ice or collect via centrifugation at ~2,000× g for 2 min; remove and save the supernatant for calculation of coupling efficiency.
Add 25 mL of cold 0.1 M Tris pH 8.0 to block unreacted sites on beads. Rock at 4 °C for 2 h.
Collect resin via centrifugation at ~2,000× g for 2 min. Wash resin, alternating between 25 mL of each of the following solutions thrice:
Low pH wash buffer.
High pH wash buffer.
Wash resin twice with 25 mL of DNase I storage buffer; resuspend in DNase I storage buffer and store at 4 °C.
Determine coupling efficiency by comparing DNase I concentration of the pre- (step B3) and post-coupling (step B7) solutions; this can be done with standard protein determination reagents [e.g., Bio-Rad protein assay reagent, Bradford reagent, Lowry reagent, or direct A280 measurement (A280 of 1 = 0.5 mg/mL DNase I)]. Coupling efficiency should be ~ 95%–98%.
Column packing: 2 × 3 mL columns
The DNase I-Sepharose resin will be packed into two Pierce polypropylene columns, assembled from two 5 mL polypropylene columns, two filter discs, two column end caps, and two column top funnels.
Using an upside-down 1 mL pipette tip, push one filter disc to the bottom of each column and pass 5 mL of ddH2O through each.
Attach the funnel/reservoir to the top of the column: use parafilm to seal potential leak points between the funnel neck and the column (Figure 1A and 1B).
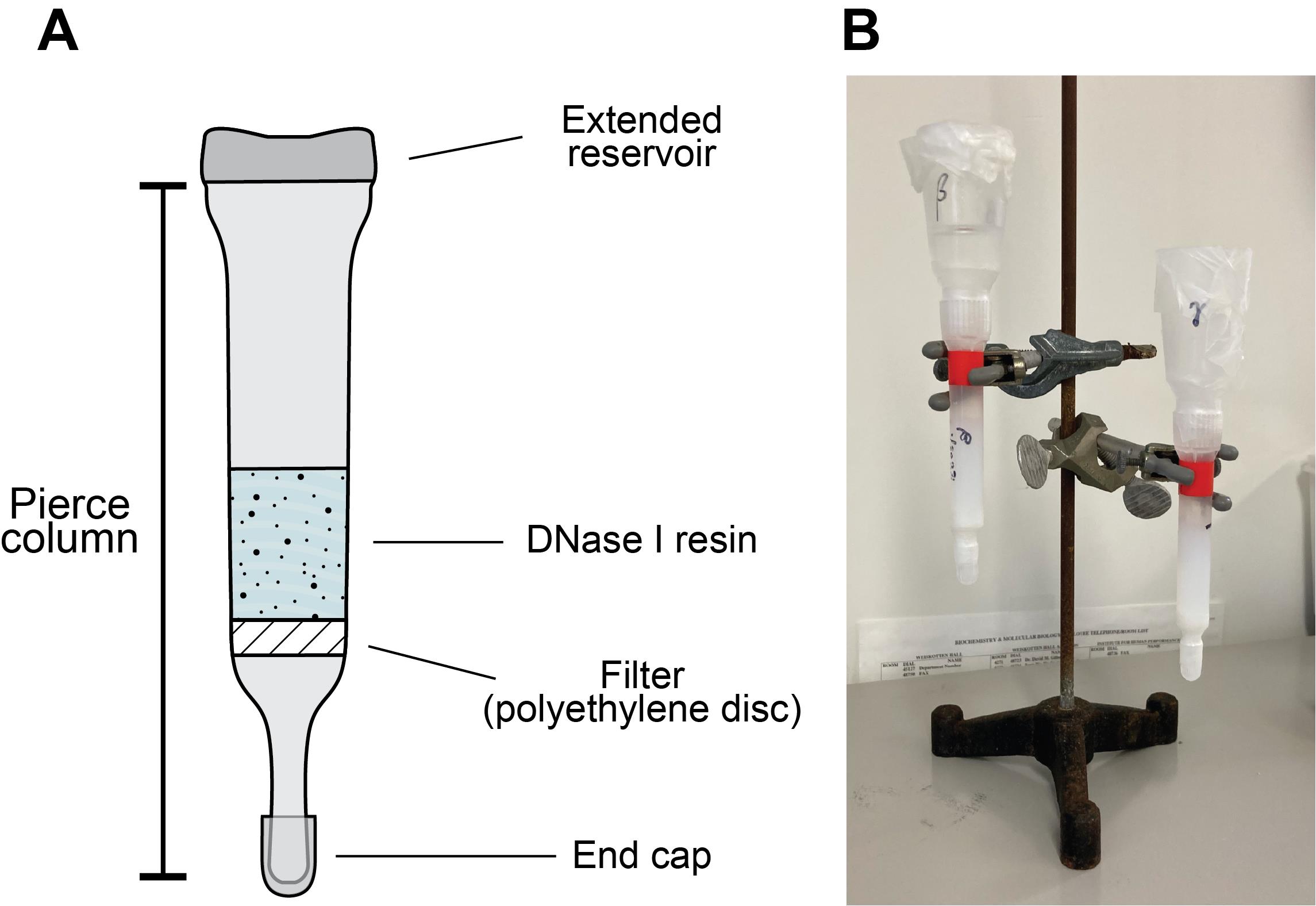
Figure 1. Diagram of disposable column assembly. (A) Schematic and (B) photo depicting column apparatus. See Protocol section D for details.Cap the columns, add 3 mL of water, and note the top water level on the column using a marker. Uncap the column and remove the water via gravity flow.
Gradually add the resin to the columns, allowing it to slowly settle via gravity as the column flows. Add enough resin to reach the 3 mL mark.
Rinse columns and reservoirs with DNase I storage buffer to settle remaining resin via gravity flow.
Wash the column with 3–5 column volumes (CV) of DNase I storage buffer; then, cap the column (e.g., by covering with parafilm) and store at 4 °C until use (Section D).
Columns are generally reusable for several (3–5) purifications. Following each purification, the column should be washed with at least five CV of G-buffer, then washed (3–5 CV) and stored in DNase I storage buffer.
Actin purification [modified from Goode (2002) and Aggeli et al. (2014)]
Thaw cell pellet obtained from 1 L media in ~15 mL of lysis buffer.
Lyse the cells by passing through French press 2× at press setting of 1,000 psi (16,000 actual psi in a pressure cell with 1-inch diameter piston).
Spin lysate at ~17,000–20,000× g for 30 min at 4 °C (e.g., in a Beckman JA20 rotor at 12 krpm).
Spin the supernatant of the previous step in a Beckman Ti70 rotor at 50,000 rpm (256,600× g) for 50 min at 4 °C.
Wash each 3 mL of DNase I-Sepharose column with 15 mL (five CV) of G-buffer supplemented with 0.2 M NH4Cl followed by 15 mL of G-buffer supplemented with 0.1 mM PMSF.
Filter the clarified supernatant through 0.45 μm syringe filters (Figure 2A, Lanes 1 and 2).
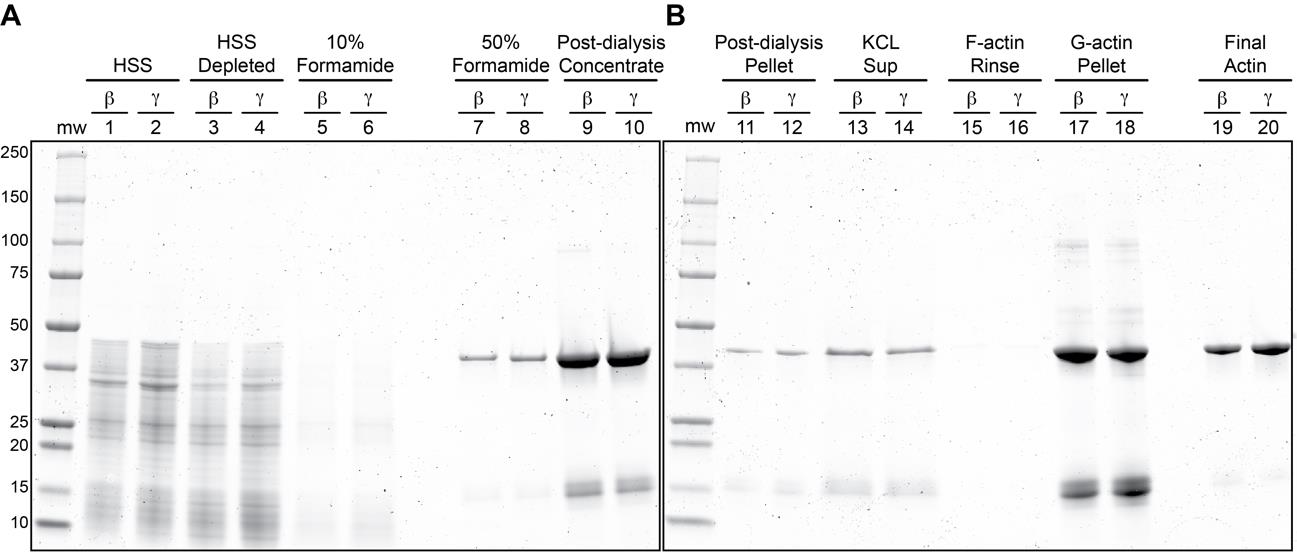
Figure 2. Coomassie stained SDS-PAGE gels of β- and γ-actin purification steps. (A–B) Two gels showing the purification steps of β- and γ-actin. Relative amounts post 50% formamide elution, as follows: Lanes 7 and 8: 1/900 of total eluates; Lanes 9 and 10: 1/100 of post-dialysis concentrated samples; Lanes 11 and 12: 1/10 of G-actin pellets (after suspension in gel loading buffer); Lanes 13 and 14: 1/75 of KCl-treated F-actin supernatants; Lanes 17 and 18: 1/10 of G-actin pellets (after suspension in gel loading buffer); Lanes 19 and 20: 1/200 of final actin preparations. Abbreviations: MW, molecular weight markers; HSS, high speed supernatant; Sup, supernatant; F-actin, filamentous actin; G-actin, globular (monomeric) actin.Load supernatants on DNase I columns and allow to drip through (Figure 2A, Lanes 3 and 4).
Note: The “depleted” flowthrough can be collected and analyzed by western blotting to determine the degree of binding and whether actin has saturated the DNase I resin.
Wash each column with:
15 mL (i.e., five CV) of actin wash buffer 1 (Figure 2A, Lanes 5 and 6).
15 mL of actin wash buffer 2.
15 mL of G-buffer.
Elute actin from each column with actin elution buffer (formamide disrupts the actin–DNase I interaction, likely denaturing one or both proteins); for 3 mL of resin, add 2 mL (<1 CV) of actin elution buffer to the column and discard the resulting flowthrough, then add another 4 mL and collect the flowthrough, dripping into a tube containing 2 mL of G-buffer (this helps to immediately reduce the formamide concentration and overall actin denaturation) (Figure 2A, Lanes 7 and 8).
Dialyze overnight against 2 L of G-buffer at 4 °C in Slide-A-Lyzer cassettes (10,000 MWCO) or dialysis tubing (12,000–14,000 MWCO); this step reduces the formamide concentration to ~0.1% and introduces fresh G-buffer into the actin solution.
Concentrate the dialyzed actin solution to ~ 0.5–1.5 mL using centrifugation-based protein concentrators (Figure 2A, Lanes 9 and 10); we have successfully used Vivaspin 5000 MWCO and Amicon Ultra 10,000 MWCO centrifugal concentrators for this step.
Distribute to 1 mL ultracentrifuge tubes and clarify actin at 213,000× g (70,000 rpm in a Beckman TLA100.2 rotor) for 30 min at 4 °C. This step removes aggregated actin (Figure 1B, Lanes 11 and 12).
Note: Larger volumes can be accommodated here and at subsequent steps using the higher capacity TLA100.3 rotor and associated tubes.
Distribute a maximum of 760 μL of the G-actin supernatant per tube to new 1 mL ultracentrifuge tubes.
Add 20× F-buffer to 1× final concentration per 1 mL ultracentrifuge tube (i.e., 40 μL of F-buffer to the 760 μL of G-actin already in the tube from step D13). Incubate tubes at room temperature for 20 min to polymerize F-actin.
Add KCl to 600 mM final (i.e., 200 μL of 3 M KCl stock to 800 μL mixture in step D14). Incubate at room temperature for 1 h. This step promotes the dissociation of potential host cofilin contaminants.
Collect F-actin via centrifugation at 213,000× g for 30 min at 20 °C. Remove and discard the supernatant (Figure 2B, lanes 13 and 14).
Rinse the F-actin pellets with ~200 μL of G-buffer (Figure 2B, Lanes 15 and16) and then resuspend in ~500 μL of G-buffer. Depolymerize actin filaments on ice overnight or for at least 3 h. Mechanically sheering actin filaments with a P200 pipette or by dounce homogenization will further expedite depolymerization.
Remove depolymerization-incompetent actin and actin aggregates via centrifugation at 213,000× g for 30 min at 4 °C (Figure 2B, lanes 17 and 18).
Transfer supernatants (G-actin) to new 1 mL ultracentrifuge tubes. Determine concentration and yield (e.g., using Bio-Rad protein assay reagent and comparing to known quantities of BSA).
Run 200–300 ng of actin on a 10%–15% polyacrylamide gel to assess purity (Figure 2B, Lanes 19 and 20).
To further enhance the purity of actin, perform 2–3 additional polymerization/depolymerization “cycles” (concentration permitting). To cycle actin, add 20× F-buffer to 1× final concentration, incubate for 20 min at room temperature, then repeat steps D16–D19 as above. Running small aliquots of the supernatants and resuspended pellets on SDS-PAGE gels can be used to track the final purity and/or loss of actin with each polymerization cycle. The amount of G-buffer to add when repeating step D17 will depend on yield and desired final concentration (e.g., suspending 500 μg of actin in 500 μL of G-buffer results in a 24 μM actin solution).
Store actin monomers in one of two ways:
For long-term storage, distribute G-actin into small (20–50 μL) aliquots, flash freeze in liquid nitrogen, and store at -80 °C.
For short-term storage (1–2 weeks), add 20× F-buffer (i.e., 5 μL of 20× F-buffer per 95 μL of G-actin) and store polymerized F-actin at 4 °C.
When ready to use:
Thaw aliquot and clarify monomers via centrifugation at 213,000× g for 30 min at 4 °C to remove aggregates.
Spin F-actin at 213,000× g for 30 min at 20 °C, resuspend actin pellet in fresh G-buffer, and incubate on ice to depolymerize (see step D17).
Validation of protocol
This protocol has been used and validated in the following research article:
Haarer et al. (2023). Purification of human β- and γ-actin from budding yeast. Journal of Cell Science 136: jcs260540. Doi: 10.1242/jcs.260540.
General notes and troubleshooting
General notes
BHY848 is respiratory incompetent (petite). We recommend growing both strains (BHY848 and BHY845) with 4% glucose.
It is convenient to make β- and γ-actin preparations in parallel. We recommend having dedicated DNase I columns for each isoform to avoid cross-contamination.
Run each step of the purification (including column washes and spin supernatants and pellets) on polyacrylamide gels to assess effectiveness, actin loss, and relative purity (see Figure 2).
DNase I affinity columns lose effectiveness over time. We recommend using columns for 3–5 purifications.
Alternate yeast lysate preparation methods can be used in the absence of a French press with pressure cell, including microfluidizer, ball mill, or bead beater.
There are many methods for purifying actin. We see no reason why alternate methods would not be compatible with purification of β- or γ-actin from clarified yeast lysates.
Growing yeast in bioreactors or generally scaling cultures > 1 L permits additional polymerization/depolymerization cycles and gel filtration methodologies.
Troubleshooting
Problem 1: Low protein yield due to cell lysis (step D2).
Possible cause: Cell lysis is incomplete.
Solutions: After passing through French press (or comparable breaking procedure), observe cells under a microscope; if many cells remain unbroken, pass through breaking procedure one or two more times.
Problem 2: DNase I binding capacity is low.
Possible causes: Low efficiency coupling of DNase I to new resin or saturation/breakdown of a reused column.
Solution: Replace column.
Problem 3: Formide-related actin denaturation.
Possible cause: The ratio of Formide to actin was too high.
Solution: Limit exposure to formamide by dilution (leaving space for solution in step D9). Keep samples on ice whenever possible.
Problem 4: Final concentration of actin is too low (step D19 onward).
Possible cause: Loss of actin during cycling.
Solutions: Ensure that F-actin pellets have had sufficient time and agitation to convert to G-actin. To maximize recovery of low-yield actin, resuspend F-actin pellets in G-buffer to at least 1 μg/μL prior to converting to F-actin.
Acknowledgments
Components of the graphical overview were created with and exported from BioRender.com under a paid subscription purchased by SUNY Upstate. This work was supported by R35GM133485 to JLH-R, and by support from SUNY Upstate Medical University and the Research Foundation of SUNY to DCA. We are grateful to Thomas J. Black for his acerbic wit, energy, and peaches. This protocol was adapted from our previously published study (Haarer et al., 2023).
Competing interests
The strains described in this protocol and their use for purifying human cytoplasmic actin isoforms are the subject of a provisional patent application by the authors and SUNY Research Foundation (App No: 63/399,088).
References
- Aggeli, D., Kish-Trier, E., Lin, M. C., Haarer, B., Cingolani, G., Cooper, J. A., Wilkens, S. and Amberg, D. C. (2014). Coordination of the filament stabilizing versus destabilizing activities of cofilin through its secondary binding site on actin. Cytoskeleton 71(6): 361–379.
- Antoku, S., Wu, W., Joseph, L. C., Morrow, J. P., Worman, H. J. and Gundersen, G. G. (2019). ERK1/2 Phosphorylation of FHOD Connects Signaling and Nuclear Positioning Alternations in Cardiac Laminopathy. Dev. Cell 51(5): 602–616.e12.
- Bamburg, J. R. and Bernstein, B. W. (2016). Actin dynamics and cofilin-actin rods in alzheimer disease.Cytoskeleton 73(9): 477–497.
- Belin, B. J., Lee, T. and Mullins, R. D. (2015). Correction: DNA damage induces nuclear actin filament assembly by Formin-2 and Spire-1/2 that promotes efficient DNA repair. eLife 4: e11935.
- Ceron, R. H., Carman, P. J., Rebowski, G., Boczkowska, M., Heuckeroth, R. O. and Dominguez, R. (2022). A solution to the long-standing problem of actin expression and purification. Proc. Natl. Acad. Sci. U. S. A. 119(41): e2209150119.
- De La Cruz, E. M. (2005). Cofilin Binding to Muscle and Non-muscle Actin Filaments: Isoform-dependent Cooperative Interactions. J. Mol. Biol. 346(2): 557–564.
- Goode, B. L. (2002). Purification of yeast actin and actin-associated proteins. Methods Enzymol. 351: 433–441.
- Haarer, B. K., Pimm, M. L., de Jong, E. P., Amberg, D. C. and Henty-Ridilla, J. L. (2023). Purification of human β- and γ-actin from budding yeast. J. Cell Sci. 136(9): e260540.
- Hatano, T., Alioto, S., Roscioli, E., Palani, S., Clarke, S. T., Kamnev, A., Hernandez-Fernaud, J. R., Sivashanmugam, L., Chapa-Y-Lazo, B., Jones, A. M. E., et al. (2018). Rapid production of pure recombinant actin isoforms in Pichia pastoris. J. Cell Sci. 131(8): jcs213827.
- Hatano, T., Sivashanmugam, L., Suchenko, A., Hussain, H. and Balasubramanian, M. K. (2020). Pick-ya actin: a method to purify actin isoforms with bespoke key post-translational modifications.J. Cell Sci. 133(2): jcs241406.
- Hyrskyluoto, A. and Vartiainen, M. K. (2020). Regulation of nuclear actin dynamics in development and disease. Curr. Opin. Cell Biol. 64: 18–24.
- Izdebska, M., Zielińska, W., Hałas-Wiśniewska, M. and Grzanka, A. (2020). Involvement of Actin and Actin-Binding Proteins in Carcinogenesis. Cells 9(10): 2245.
- Kadzik, R. S., Homa, K. E. and Kovar, D. R. (2020). F-Actin Cytoskeleton Network Self-Organization Through Competition and Cooperation. Annu. Rev. Cell Dev. Biol. 36(1): 35–60.
- Kilmartin, J. V. and Adams, A. E. (1984). Structural rearrangements of tubulin and actin during the cell cycle of the yeast Saccharomyces. J. Cell Biol. 98(3): 922–933.
- Kron, S. J., Drubin, D. G., Botstein, D. and Spudich, J. A. (1992). Yeast actin filaments display ATP-dependent sliding movement over surfaces coated with rabbit muscle myosin. Proc. Natl. Acad. Sci. U. S. A. 89(10): 4466–4470.
- Perrin, B. J. and Ervasti, J. M. (2010). The actin gene family: function follows isoform. Cytoskeleton 67(10): 630–634.
- Rivière, J. B., van Bon, B. W. M., Hoischen, A., Kholmanskikh, S. S., O’Roak, B. J., Gilissen, C., Gijsen, S., Sullivan, C. T., Christian, S. L., Abdul-Rahman, O. A., et al. (2012). De novo mutations in the actin genes ACTB and ACTG1 cause Baraitser-Winter syndrome. Nat. Genet. 44(4): 440–444.
- Rubenstein, P. A. and Wen, K. K. (2014). Insights into the effects of disease-causing mutations in human actins.Cytoskeleton 71(4): 211–229.
- Schafer, D. A., Jennings, P. B. and Cooper, J. A. (1998). Rapid and efficient purification of actin from nonmuscle sources. Cell Motil. Cytoskeleton 39(2): 166–171.
- Schaks, M., Giannone, G. and Rottner, K. (2019). Actin dynamics in cell migration. Essays Biochem. 63(5): 483–495.
- Zechel, K. (1980). Isolation of Polymerization-Competent Cytoplasmic Actin by Affinity Chromatography on Immobilized DNAse I Using Formamide as Eluant. Eur. J. Biochem. 110(2): 343–348.
Article Information
Publication history
Published: Dec 5, 2023
Copyright
© 2023 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
How to cite
Haarer, B. K., Amberg, D. C. and Henty-Ridilla, J. L. (2023). Purification of Human Cytoplasmic Actins From Saccharomyces cerevisiae. Bio-protocol 13(23): e4894. DOI: 10.21769/BioProtoc.4894.
Category
Microbiology > Heterologous expression system > Saccharomyces cerevisiae
Biochemistry > Protein > Isolation and purification
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Tips for asking effective questions
+ Description
Write a detailed description. Include all information that will help others answer your question including experimental processes, conditions, and relevant images.
Share
Bluesky
X
Copy link