- Submit a Protocol
- Receive Our Alerts
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Cotton Ovules Culture and Analysis
Published: Vol 3, Iss 22, Nov 20, 2013 DOI: 10.21769/BioProtoc.972 Views: 10007
Reviewed by: Tie Liu

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Quantification of Ethylene Production in Leaf and Bud Tissue of the Subtropical Tree Crop Litchi (Litchi chinensis Sonn.) Using Gas Chromatography and Flame Ionization Detection
Regina B. Cronje and Arnoldus J. Jonker
Mar 20, 2023 421 Views
Modified Pseudo-Schiff Propidium Iodide for Staining the Shoot Apical Meristem in Arabidopsis
Ruiqi Li [...] Ligeng Ma
May 5, 2023 525 Views
A Novel Imaging Protocol for Investigating Arabidopsis thaliana Siliques and Seeds Using X-rays
Brylie A. Ritchie [...] Ansul Lokdarshi
Oct 5, 2023 685 Views
Abstract
The cotton ovules culture was innovated by Beasley and Ting (1973), and named after them. It is a convenient system to analyze the effect of chemical or environmental treatment on fiber development directly on ovules. This protocol was generated according to previous published papers and our practical experience.
Keywords: CottonMaterials and Reagents
- Flowers (-1 to 1 DPA of flowers are easy to manipulate)
- 0.1% (w/v) HgCl2 (caution: HgCl2 is very dangerous. Please select an advantageous reagent to sterilize according to your lab condition. Otherwise 75% ethanol and N,N'-Dicyclohexylcarbodiimide (DCCS) could be the alternatives)
- Toluidine blue O (Sigma-Aldrich, catalog number: T3260 )
- Glacial acetic acid-ethanol-water (10:95:5, v/v)
- Vitamins:
VB1(Vitamin B1, Thiamine) (Sigma-Aldrich, catalog number: T3902 )
VB6 (Vitamin B6, Pyridoxine) (Sigma-Aldrich, catalog number: P8666 )
VB3 (Vitamin B3, Nicotinic acid) (Sigma-Aldrich, catalog number: N0765 ) - Inositol (Sigma-Aldrich, catalog number: I3011 )
- IAA (Sigma-Aldrich, catalog number: I2886 )
- GA3 (Sigma-Aldrich, catalog number: G7645 )
- KH2PO4 (Sigma-Aldrich)
- CaCl2.2H2O (Sigma-Aldrich)
- MgSO4.7H2O (Sigma-Aldrich)
- KNO3 (Sigma-Aldrich)
- H3BO3 (Sigma-Aldrich)
- Na2MoO4.2H2O (Sigma-Aldrich)
- KI (Sigma-Aldrich)
- CoCl2.6H2O (Sigma-Aldrich)
- MnSO4.H2O (Sigma-Aldrich)
- ZnSO4.7H2O (Sigma-Aldrich)
- CuSO4.5H2O (Sigma-Aldrich)
- FeSO4.7H2O (Sigma-Aldrich)
- Na2EDTA (Sigma-Aldrich)
- BT medium preparation (see Recipes)
- Hormone preparation (see Recipes)
- Working BT medium preparation (see Recipes)
Equipment
- Erlenmeyer flask (50 ml)
- 1.5 ml microcentrifuge tubes
- Spectrophotometer (Beckman Coulter, model: DU 800 ) or microplate reader (Tecan Trading AG, model: infinite® M200 )
- Clean bench laminar air-flow-hood with burner (Harbin East Electronic Technology, model: HD-1360 )
- Common plant tissue culture equipment (Measuring cylinder/volumetric flask, Beaker, Weighing machine, Autoclave, pH-meter, Scalpel, Fine forceps)
Software
- ImageJ
Procedure
- Ovary sterilization
- Flowers (Figure 1) are collected with 1 to 3 cm anthocaulus from plants (the length of anthocaulus is usually 1 to 3 cm in our used cotton species, if some others is longer, you can cut as you wish; but the shorter ones is not recommended to choose unless there is no alternative). Remove the petals, stamens and bracts with hand carefully and thoroughly (or you can do it with some special accessory appliances but make sure do not hurt the cotton boll).
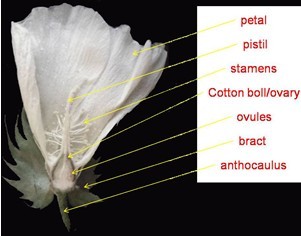
Figure 1. The organ of cotton flower - If the pistils are vigorous, such as those from in or before blooming flowers in which the pistils are linked with the cotton boll, while they will be physiological abscission after 2 day post anthesis (DPA), they should be kept to avoid liquid permeating into the cotton boll through the wound when sterilized.
- Soak the whole remaining tissue (cotton boll linked with anthocaulus and/or pistils) into 0.1% (w/v) HgCl2 for 15 min immediately (occasional shaking/stirring is needed to keep all the tissue fully soaked), discard HgCl2 and rinse with sterilized ddH2O three times. Each for 2 to 5 min.
- Flowers (Figure 1) are collected with 1 to 3 cm anthocaulus from plants (the length of anthocaulus is usually 1 to 3 cm in our used cotton species, if some others is longer, you can cut as you wish; but the shorter ones is not recommended to choose unless there is no alternative). Remove the petals, stamens and bracts with hand carefully and thoroughly (or you can do it with some special accessory appliances but make sure do not hurt the cotton boll).
- Ovules preparation
- Hold the tissue with the anthocaulus, and remove the shuck of the ovary (or the young cotton boll) with a sterilized forceps carefully, then float and disperse the intact ovules on the liquid BT medium gently. Make sure the ovules are not injured, broken or attached the brown gossypol from the broken shuck.
- For same batch culture, equal ovules should be put in each flask and usually less than 20 ovules for each batch.
- If the ovules are going to culture for more than 10 days, the ovules sum in each flask should be less than 10 at the beginning.
- For young ovules that have not grown fiber, they could be separated easily, while for ovules with longer fiber that are sticking together, such as the ovules in 2 day post anthesis (DPA), they need to be carefully separated one by one with forceps before floating on the medium.
- Label the flask with the date, the age of the ovules and the hormones contained.
- Hold the tissue with the anthocaulus, and remove the shuck of the ovary (or the young cotton boll) with a sterilized forceps carefully, then float and disperse the intact ovules on the liquid BT medium gently. Make sure the ovules are not injured, broken or attached the brown gossypol from the broken shuck.
- Ovule culture
- The floating ovules are incubated in 30 °C without light and shake.
- The fiber should be easily visible after 4-5 days of culture for the 0 DPA ovules (Figure 2).
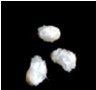
Figure 2. 0 DPA ovules after 5 days culture - The ovules or fiber can be used for further analysis, such as fiber production measurement, RNA extraction and biochemical analysis etc.
- The floating ovules are incubated in 30 °C without light and shake.
- Fiber measurement
There are two methods to measure fiber production.- Total fiber units (TFU) measurement, which was also innovated by Beasley et al. (1974).
- For 12 days cultured ovules, 10 ovules for each assay. Ovules are dried out of medium with filter paper. Sunk in boiling water for 2 min, then dried again with filter paper for 2 to 3 min.
- Stain the ovules in a small beaker with 20 ml 0.02% Toluidine blue O for 30 sec, then discard the liquid with a fine sieve and wash in running water for 1 min immediately to remove the non-absorbed dye.
- Dry the ovules, then destain in flask with 20 ml glacial acetic acid-ethanol-water (10:95:5) for 2 h. The flask should be sealed to avoid liquid evaporation which can cause differences in concentration of destaining liquid.
- Measure the absorbance of the destaining liquid in 624 nm and count as the relative yield of fiber.
- For 12 days cultured ovules, 10 ovules for each assay. Ovules are dried out of medium with filter paper. Sunk in boiling water for 2 min, then dried again with filter paper for 2 to 3 min.
- Fiber length measurement (Figure 3)
- Ovules are sunk in boiling water for 2 min or in 75% ethanol for 15 min, then put the ovules in slowly running water to make the fiber flow to one side and measured with a ruler.
- Or put the ovules on a glass slide, comb the fiber gently with a dissecting needle, then take photos and measure the length with a ruler or you can use software for automation of the measurement (e.g. Image J).

Figure 3. Cultured ovule (1) is soaked in 75% ethanol (2) to separete fiber and measured on a glass slide (3)
- Ovules are sunk in boiling water for 2 min or in 75% ethanol for 15 min, then put the ovules in slowly running water to make the fiber flow to one side and measured with a ruler.
- Total fiber units (TFU) measurement, which was also innovated by Beasley et al. (1974).
Notes
- This protocol can be used for analyzing the effects of dozen of chemicals, including hormones, plant growth regulators, plant growth inhibitors and flavonoids (Tan et al., 2013) etc., on fiber development in our lab. We found it functioned similarly in Gossypium hirsutum (more than 4 cultivars were applied), G. arboreum and G.herbaceum. But for G. barbadense, there will be several differences in the fiber growth rate and hormones response. The fiber will grow later and fewer, and GA3 can’t promote fiber grow for the 0 DPA ovules independently.
- While it is a very sensitive system, many factors are involved in a success assay, especially for that of ovule developmental stage. According to our unpublished data, there are dramatic differences for 0 DPA ovules from 7 am to 12 am in response to IAA and GA3. And GA3 could promote fiber growth independently on the 0 DPA ovules post 12 am except for that from G. barbadense. The ovules before 0 DPA may be not easy to float on the liquid medium, should be taken more gently. And ovules of different developmental stages are also response differentially to chemical treatment (Tan et al., 2012). So for the same assay, the ovules should be from the same stage and all the manipulations should be strictly consistent and control should be set up for each. The samples from different batches could not analyze together.
- CaCl2.2H2O should be separately prepared and stored with KH2PO4 and MgSO4.7H2O, be carefully mixed and diluted before use.
- Other chemical should be sterilized first and added into the medium with IAA and/or GA3. Each new chemical should be undergone a dose test to determine the optimal dose.
- Infection rate of ovules from the glasshouse is less than that from the field.
- Take a simple plan before start. It will take a lot of time to release the ovules from the ovary (approximately 10 ovules per hour for freshman).
- For TFU assay, it is 10 ovules for each assay usually, while it should be changed depending on the days of culture and the bulk of ovules.
Recipes
- BT medium preparationPreparation of 20x Macro element stock solution(stored in 4 °C)
Chemicals (company) Mol.wt Final conc.(mg/L)Final conc.(mM)Conc. stock(mM)mg to take Final volume of stock KH2PO4 136.09 272.18 2 40 5,443.6 1,000 ml CaCl2.2H2O 147.01 441.06 3 60 8,821.2 MgSO4.7H2O 246.47 493.00 2 40 9,860 KNO3 101.10 5,055.50 50 1,000 101,110 Preparation of 100x Micro element stock solution(stored in 4 °C)H3BO3 61.83 6.183 0.1 10 618.3 1,000 ml Na2MoO4.2H2O 241.95 0.242 0.001 0.1 24.2 KI 166 0.830 0.005 0.5 83 CoCl2.6H2O 237.93 0.024 0.0001 0.01 2.4 MnSO4.H2O 169.02 16.902 0.1 10 1,690.2 ZnSO4.7H2O 287.56 8.627 0.03 3 862.7 CuSO4.5H2O 249.69 0.025 0.0001 0.01 2.5 Preparation of 100x Fe salt stock solution(stored in brown bottle and 4 °C)FeSO4.7H2O 278.01 8.341 0.03 3 834.1 1,000 ml Na2EDTA 372.24 11.167 0.03 3 1,116.7 Preparation of 1,000x Vitamins mixture stock solution(stored in 4 °C)VB1 337.27 1.349 0.004 4 1,349 1,000 ml VB6 205.64 0.822 0.004 4 822 VB3 123.11 0.492 0.004 4 492 Preparation of 100x Inositol stock solution(stored in 4 °C)Inositol 180.16 180.160 1 100 18,016 1,000 ml - Hormone preparing
The general hormones for cotton ovules culture are IAA and GA3.The stock solutions of IAA and GA3 are 5 mM and 0.5 mM, respectively. Both are 1,000x solutions, pre-dissolved in a small volume (500-1,000 μl) of 95% ethyl alcohol, then brought to volume with sterilized double-distilled H2O water, aliquoted into 1.5 ml microcentrifuge tubes, sealed and stored in -20 °C. Usually 10 to 50 ml stock solution is prepared for each time. - Working BT medium preparation
Stock Conc. of stock Amount of stock soln to take Final volume of media Macro-element mixture 20x 50 ml 1,000 ml Micro-element mixtures 100x 10 ml Fe salt solution 100x 10 ml Vitamin mix 1,000x 1 ml Inositol 100x 10 ml Glucose 24 g pH 5.0
Medium is prepared in 100, 200 ml or other certain volumes before used, after sterilized, added suitable hormones, mixed and aliquoted into sterilized 50 ml flasks with about 10 ml each. Label the flask with what kinds of hormones contained, preparation and expiration dates (the medium should be used within less than 15 days).
Acknowledgments
The protocol was based on Beasley and Ting’s original work (Beasley and Ting, 1973), and adapted from two of our previous published work (Tan et al., 2012; Tan et al., 2013). This work was supported by the University Scientific and Technological Self-innovation Foundation, the National Natural Science Foundation of China (grant no. 30871560 and 31230056) and the National High-Tech Program of China (grant no. 2012AA101108).
References
- Beasley, C. and Ting, I. P. (1973). The effects of plant growth substances on in vitro fiber development from fertilized cotton ovules. Am J Bot 130-139.
- Beasley, C. A., Birnbaum, E. H., Dugger, W. M. and Ting, I. P. (1974). A quantitative procedure for estimating cotton fiber growth. Stain Technol 49(2): 85-92.
- Tan, J., Tu, L., Deng, F., Hu, H., Nie, Y. and Zhang, X. (2013). A genetic and metabolic analysis revealed that cotton fiber cell development was retarded by flavonoid naringenin. Plant Physiol 162(1): 86-95.
- Tan, J., Tu, L., Deng, F., Wu, R. and Zhang, X. (2012). Exogenous jasmonic acid inhibits cotton fiber elongation. J Plant Growth Regul 31(4): 599-605.
Article Information
Copyright
© 2013 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Tan, J., Deng, F., Tang, W., Han, J., Kai, G., Tu, L. and Zhang, X. (2013). Cotton Ovules Culture and Analysis. Bio-protocol 3(22): e972. DOI: 10.21769/BioProtoc.972.
-
Tan, J., Tu, L., Deng, F., Hu, H., Nie, Y. and Zhang, X. (2013). A genetic and metabolic analysis revealed that cotton fiber cell development was retarded by flavonoid naringenin. Plant Physiol 162(1): 86-95.
Category
Plant Science > Plant developmental biology > Morphogenesis
Plant Science > Plant physiology > Plant growth
Plant Science > Plant cell biology > Tissue analysis
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Tips for asking effective questions
+ Description
Write a detailed description. Include all information that will help others answer your question including experimental processes, conditions, and relevant images.
Share
Bluesky
X
Copy link