- Submit a Protocol
- Receive Our Alerts
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Assay to Evaluate Vascular Permeability Induction in Mice
Published: Vol 3, Iss 22, Nov 20, 2013 DOI: 10.21769/BioProtoc.977 Views: 21654
Reviewed by: Anonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

TCRβ Clonotype Analysis of EBV and CMV-specific Human CD8+ T Cells
Nening M. Nanlohy [...] Debbie van Baarle
Oct 5, 2015 7582 Views
Multicolor Stimulated Emission Depletion (STED) Microscopy to Generate High-resolution Images of Respiratory Syncytial Virus Particles and Infected Cells
Masfique Mehedi [...] Ursula J. Buchholz
Sep 5, 2017 8678 Views
A Triple-challenge Mouse Model of Allergic Airway Disease, Primary Influenza Infection, and Secondary Bacterial Infection
Sean Roberts [...] Yoichi Furuya
Apr 20, 2020 3064 Views
Abstract
Dengue virus infection usually courses as a benign self-limited fever, called dengue fever. However, on occasions it can progress to a life-threatening complication known as severe dengue (SD). A hallmark of SD is a sharp increase in vascular permeability. Secondary infections are considered a risk factor to develop SD, presumably through a mechanism called Antibody-Dependent Enhancement (ADE) of infection in cells with the capacity to bind antigen-antibody complexes, such as macrophages, and to trigger a subsequent aberrant cytokine response. The massive release of cytokine from macrophages has been postulated to cause changes in vascular permeability. The vascular permeability assay presented in this protocol is designed to assess whether any compound or cell-secreted product or soluble factor present in sera from patients may induce plasma leakage in mice. This test was used in the laboratory to determine whether cytokines and soluble factors produced in vitro by macrophages infected with dengue virus or dengue virus in the presence of facilitating antibodies are able to induce plasma leakage in vivo. Macrophages were infected with dengue virus or dengue virus in the presence of facilitating antibodies for 48 h. After this time, the conditioned supernatant containing cytokines and soluble factors released by the macrophages were collected and inoculated intraperitoneally into CD-1 mice. Twenty four hours after the first inoculation, mice were reinoculated with a second dose with Evans blue dye. After another 24 h, mice were euthanized and the amount of Evans blue present in the blood and lung was determined by spectrophotometric analysis. The assay was able to show differences in the capacity of the conditioned media to induce vascular permeability changes in the inoculated animals (Puerta-Guardo et al., 2013).
Keywords: DengueMaterials and Reagents
- Mice of six to seven weeks old (CD-1® Mouse, Crl:CD1 (ICR)) (Charles River Laboratories)
- Evans Blue Dye (Sigma-Aldrich, catalog number: E2129 )
- Ketamine (Sigma-Aldich, catalog number: K2753 )
- Xylazine (Sigma-Aldich, catalog number: X1251 )
- TNF-α (BD CBA flex set) (BD Biosciences, catalog number: 558273 )
- 10% Formalin (Sigma-Aldich, catalog number: HT501128 )
- Formamide (Sigma-Aldich, catalog number: F8775 )
- NaCl
- KCl
- Na2HPO4
- KH2PO4
- HCl
- 1x PBS (see Recipes)
- 1% (w/v) Evans blue dye solution in PBS (see Recipes)
Equipment
- EDTA tubes
- Lyophilizer
- Centrifuge (Beckman Coulter, model: Allegra X-12R ) (Rotor SX4750)
- Spectrophotometry (BioTek Instruments, model: ELx808 Absorbance Microplate Reader)
Procedure
- Mice of six to seven weeks old and 18-20 g in weight in a minimal amount of 4 mice per group, were injected intraperitoneally with 2 doses (100 μl each) 24 h apart, of media or supernatants to be evaluated.
- Since TNF-α has been described as a plasma leakage inductor, this cytokine can be used for the injection as positive control (4 ng/ml).
- Together with the second dose of conditioned supernatant, mice were injected intraperitoneally with 4 ml/kg of weight a 1% Evans blue dye solution (w/v in PBS) allowing circulation for 24 h as was described previously (Manaenko et al., 2011).
- Before samples were collected, mice were injected with anesthetics: ketamine (70 mg/kg) and xylazine (6 mg/kg), afterward blood samples were collected by cardiac puncture.
- Blood was immediately placed in EDTA tubes.
- Samples were diluted 1:2 v/v in PBS and plasma was separated by centrifugation at 3,000 rpm for 8 min.
- The amount of Evans blue dye present in plasma was measured by spectrophotometry at 630 nm. Quantification was performed by comparison with a standard curve.
- The standard curve was constructed using dilution of Evans blue dye in 1x PBS in a range from 3 to 400 ng/ml (R = 0.999).
- To evaluate the pulmonary capillary leakage, the mice were perfused transcardially with 50 ml of PBS followed by 20 ml of 10% Formalin for tissue fixation. The lung tissues were removed and preserved in formalin solution at 4 °C until analysis.
- The right lung was vacuum-dried, weight and frozen instantly in liquid nitrogen.
- Evans blue dye was extracted from the lung by incubation at 65 °C with formamide (2 ml/g tissue) overnight (Peng et al., 2004).
- The lung tissue was pelleted by centrifugation (12,000 x g for 30 min), and the concentration of Evans blue dye extracted in the supernatant was determined spectrophotometrically at 630 nm against a standard curve. Concentration of Evans blue dye was determined in ng/ml of lung tissue. However, this study was designed to compare the permeability induction capacity of different cell supernatants, thus, results were expressed as fold increases in relation to the mock or control condition. Nevertheless, results can also be expressed directly in ng/ml of tissue. Any statistically significant increase between the control and the experimental conditions is considered as leakage induction (Figure 1).
Note: This type of studies has to be conducted in accordance with official guidelines for the Standard Production, Care and Use of Laboratory Animals of each country.
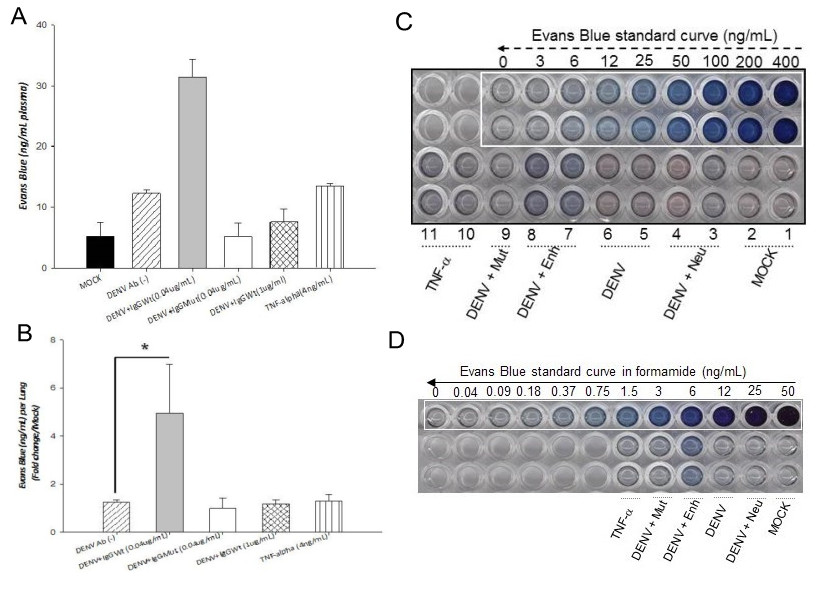
Figure 1. Effect of conditioned supernatants on vascular permeability in vivo. Six to eight weeks old mice (CD1 strain) were injected twice intraperitoneally, 24 h apart, with conditioned supernatans. A. Solution of 1% Evans Blue dye (EVD) was injected together with the second dose. Twenty four hours later, EVD was extracted from plasma and lungs and quantified against standard curves. Values are expressed as ng/ml per plasma (panel A) or as fold increases per lung in relation to the control (panel B). Panels C and D, standard curves for EVD extracted from plasma and lungs, respectively. Four mice were included per condition. Statistical significance was *p < 0.05 and **p < 0.001. Mock: supernatant collected from mock infected cells. DENV: Supernatant collected from cells infected directly with dengue virus. DENV + Enh: Supernatant collected from cells infected in the presence of enhancing antibodies. DENV + Mut: Supernatant collected from cells infected in the presence of mutated antibodies (incapable of inducing enhancing). DENV+Neu: Supernatant collected from cells infected in the presence of neutralizing antibodies. TNF-α: TNF-α used as positive control.
Recipes
- 1x PBS
8 g (137 mM) NaCl
0.2 g (2.7 mM) KCl
1.44 g (10 mM) Na2HPO4
0.24 g (2 mM) KH2PO4
Add 800 ml H2O
Adjust pH to 7.4 with HCl
Add water up to 1 L
- 1% (w/v) Evans blue dye solution in PBS
Add 1 g of Evans blue dye to 100 ml of 1x PBS
Acknowledgments
This protocol was adapted from Puerta-Guardo et al. (2013). The study was partially supported by grants 103783 and 127447 from The Mexican Council for Science and Technology (CONACYT) to JEL and RMDA, respectively. HPG and ARS are recipients of CONACYT scholarships. Authors declare no conflict of interest.
References
- Manaenko, A., Chen, H., Zhang, J. H. and Tang, J. (2011). Comparison of different preclinical models of intracerebral hemorrhage. Acta Neurochir Suppl 111: 9-14.
- Peng, X., Hassoun, P. M., Sammani, S., McVerry, B. J., Burne, M. J., Rabb, H., Pearse, D., Tuder, R. M. and Garcia, J. G. (2004). Protective effects of sphingosine 1-phosphate in murine endotoxin-induced inflammatory lung injury. Am J Respir Crit Care Med 169(11): 1245-1251.
- Puerta-Guardo, H., Raya-Sandino, A., Gonzalez-Mariscal, L., Rosales, V. H., Ayala-Davila, J., Chavez-Mungia, B., Martinez-Fong, D., Medina, F., Ludert, J. E. and del Angel, R. M. (2013). The cytokine response of U937-derived macrophages infected through antibody-dependent enhancement of dengue virus disrupts cell apical-junction complexes and increases vascular permeability. J Virol 87(13): 7486-7501.
Article Information
Copyright
© 2013 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Puerta-Guardo, H., Raya-Sandino, A., González-Mariscal, L., Rosales, V. H., Ayala-Dávila, J., Chávez-Mungía, B., Martínez-Fong, D., Medina, F., Ludert, J. E. and Angel, R. M. D. (2013). Assay to Evaluate Vascular Permeability Induction in Mice. Bio-protocol 3(22): e977. DOI: 10.21769/BioProtoc.977.
Category
Microbiology > Microbe-host interactions > In vivo model
Immunology > Immune cell function > Cytokine
Cell Biology > Cell staining > Whole cell
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Tips for asking effective questions
+ Description
Write a detailed description. Include all information that will help others answer your question including experimental processes, conditions, and relevant images.
Share
Bluesky
X
Copy link









